Abstract
In this study, an Fe2O3 photoelectrode was grown on a fluorine-doped tin oxide substrate via microwave chemical bath deposition. We added various amounts of urea as an additive to the FeCl3 precursor for the fabrication of the Fe2O3 photoelectrode and investigated the effects of the concentration of the urea additive on the morphological, optical, structural, electrical, and photoelectrochemical properties of this photoelectrode. Among the different concentrations evaluated, the maximum photocurrent density (0.51 mA/cm2 at 0.6 V vs. SCE) was obtained using 0.05 M urea, as the resulting electrode had the greatest thickness, highest flat-band potential, and preferential growth along the (110) plane along with favorable electron transport characteristics. The maximum photocurrent density of the sample prepared with 0.05 M urea was approximately 60% greater than that obtained from the sample prepared in the absence of urea. This study showed that the photoelectrochemical properties of the Fe2O3 photoelectrode were substantially influenced by the changes in the morphological, optical, structural, and electrical properties caused by the addition of urea.
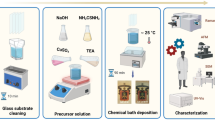
Graphic Abstract








Similar content being viewed by others
References
Chiang, C.Y., Epstein, J., Brown, A., Munday, J.N., Culver, J.N., Ehrman, S.: Biological templates for antireflective current collectors for photoelectrochemical cell applications. Nano Lett. 12, 6005–6011 (2012)
Bak, T., Nowotny, J., Rekas, M., Sorrell, C.C.: Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrog. Energy 27, 991–1022 (2002)
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972)
Zhao, D., Yang, C.F.: Recent advances in the TiO2/CdS nanocomposite used for photocatalytic hydrogen production and quantum-dot-sensitized solar cells. Renew. Sustain. Energy Rev. 54, 1048–1059 (2016)
Krbal, M., Sopha, H., Podzemna, V., Das, S., Prikryl, J., Macak, J.M.: TiO2 nanotube/chalcogenide-based photoelectrochemical cell: nanotube diameter dependence study. J. Phys. Chem. C 121, 6065–6071 (2017)
Yilmaza, C., Unal, U.: Effect of Zn(NO3)2 concentration in hydrothermal–electrochemical deposition on morphology and photoelectrochemical properties of ZnO nanorods. Appl. Surf. Sci. 368, 456–463 (2016)
Holi, A.M., Zainal, Z., Talib, Z.A., Lim, H.N., Yap, C.C., Chang, S.K., Ayal, A.K.: Hydrothermal deposition of CdS on vertically aligned ZnO nanorods for photoelectrochemical solar cell application. J. Mater. Sci. Mater. Electron. 27, 7353–7360 (2016)
Steier, L., Cardona, I.H., Gimenez, S., Santiago, F.F., Bisquert, J., Tilley, S.D., Grätzel, M.: Understanding the role of underlayers and overlayers in thin film hematite photoanodes. Adv. Funct. Mater. 24, 7681–7688 (2014)
Liu, S., Zheng, L., Yu, P., Han, S., Fang, X.: Novel composites of α-Fe2O3 tetrakaidecahedron and graphene oxide as an effective photoelectrode with enhanced photocurrent performances. Adv. Funct. Mater. 26, 3331–3339 (2016)
Brillet, J., Gratzel, M., Sivula, K.: Decoupling feature size and functionality in solution-processed, porous hematite electrodes for solar water splitting. Nano Lett. 10, 4155–4160 (2010)
Taffa, D.H., Hamm, I., Dunkel, C., Sinev, I., Bahnemann, D., Wark, M.: Electrochemical deposition of Fe2O3 in the presence of organic additives: a route to enhanced photoactivity. RSC Adv. 5, 103512–103522 (2015)
Quinn, R.K., Nasby, R.D., Baughman, R.J.: Photoassisted electrolysis of water using single crystal α-Fe2O3 anodes. Mater. Res. Bull. 11, 1011–1017 (1976)
Bjorkstbn, U., Moser, J., Gratzel, M.: Photoelectrochemical studies on nanocrystalline hematite films. Chem. Mater. 6, 858–863 (1994)
Song, X.M., Zhou, X., Yuan, C., Zhang, Y., Tong, Q., Li, Y., Cui, L., Liu, D., Zhang, W.: One-dimensional Fe2O3/TiO2 photoelectrode and investigation of its photoelectric properties in photoelectrochemical cell. Appl. Surf. Sci. 397, 112–118 (2017)
Ng, K.H., Minggu, L.J., Mark-Lee, W.F., Arifin, K., Jumali, M.H.H., Kassim, M.B.: A new method for the fabrication of a bilayer WO3/Fe2O3 photoelectrode for enhanced photoelectrochemical performance. Mater. Res. Bull. 98, 47–52 (2018)
Katsuki, H., Komarneni, S.: Role of α-Fe2O3 morphology on the color of red pigment for porcelain. J. Am. Ceram. Soc. 86, 183–185 (2003)
Dong, Q., Yin, S., Guo, C.S., Li, H.H., Kumada, N., Takei, T., Yonesaki, Y., Kinomura, N., Sato, T.: Preparation of α-Fe2O3 particles with controlled shape and size via a facile hydrothermal route. J. Phys. Conf. Ser. 339, 012004 (2012)
Dutrizac, J.E., Riveros, P.A.: The precipitation of hematite from ferric chloride media at atmospheric pressure. Metall. Mater. Trans. B 30B, 993–1001 (1999)
Virtanen, S., Schmuki, P., Davenport, A.J., Vitus, C.M.: Dissolution of thin iron oxide films used as models for iron passive films studied by in situ X-ray absorption near-edge spectroscopy. J. Electrochem. Soc. 144, 198–204 (1997)
Liu, Q., Chen, C., Yuan, G., Huang, X., Lü, X., Cao, Y., Li, Y., Hu, A., Lu, X., Zhu, P.: Morphology-controlled α-Fe2O3 nanostructures on FTO substrates for photoelectrochemical water oxidation. J. Alloys Compd. 715, 230–236 (2017)
Einert, M., Ostermann, R., Weller, T., Zellmer, S., Garnweitner, G., Smarsly, B.M., Marschall, R.: Hollow α-Fe2O3 nanofibres for solar water oxidation: improving the photoelectrochemical performance by formation of α-Fe2O3/ITO-composite photoanodes. J. Mater. Chem. A 4, 18444–18456 (2016)
Ishaq, S., Sikora, A., Scheidler, N., Hambleton, C., Katz, J.E.: Enhancement of water oxidation photocurrent for hematite thin films electrodeposited with polyvinylpyrrolidone. J. Electrochem. Soc. 163, F1330–F1336 (2016)
Su, M., He, C., Shih, K.: Facile synthesis of morphology and size-controlled α-Fe2O3 and Fe3O4 nano-and microstructures by hydrothermal/solvothermal process: the roles of reaction medium and urea dose. Ceram. Int. 42, 14793–14804 (2016)
Singh, B.P., Sharma, N., Kumar, R., Kumar, A.: Simple hydrolysis synthesis of uniform rice-shaped β-FeOOH nanocrystals and their transformation to α-Fe2O3 microspheres. Indian J. Mater. Sci. 2015, 7 (2015)
Chaudhari, N.K., Kim, H.C., Sonc, D., Yu, J.S.: Easy synthesis and characterization of single-crystalline hexagonal prism-shaped hematite α-Fe2O3 in aqueous media. CrystEngComm 11, 2264–2267 (2009)
Su, J., Feng, X., Sloppy, J.D., Guo, L., Grimes, C.A.: Grown directly on transparent conducting oxide coated glass: synthesis and photoelectrochemical properties. Nano Lett. 11, 203–208 (2011)
Song, H., Li, N., Yu, S.: Template-free synthesis of α-Fe2O3 microcubes and their magnetic property. Micro Nano Lett. 5, 200–202 (2010)
Cheng, B., Wang, W., Shi, L., Zhang, J., Ran, J., Yu, H.: One-pot template-free hydrothermal synthesis of monoclinic BiVO4 hollow microspheres and their enhanced visible-light photocatalytic activity. Int. J. Photoenergy. 2012, 1–10 (2012)
Yu, J., Kudo, A.: Effects of structural variation on the photocatalytic performance of hydrothermally synthesized BiVO4. Adv. Funct. Mater. 16, 2163–2169 (2006)
Zhang, X., Chen, Y., Liu, H., Wei, Y., Wei, W.: Facile synthesis of α-Fe2O3 hollow sub-microstructures, morphological control and magnetic properties. CrystEngComm 15, 6184–6190 (2013)
Deng, C., Hu, H., Ge, X., Han, C., Zhao, D., Shao, G.: One-pot sonochemical fabrication of hierarchical hollow CuO submicrospheres. Ultrason. Sonochem. 18, 5 (2011)
Yan, M., Qi, Z.F., Li, X.D., Chen, T., Lu, S.Y., Buekens, A.G., Olie, K., Yan, J.H.: Chlorobenzene formation from fly ash: effect of moisture, chlorine gas, cupric chloride, urea, ammonia, and ammonium sulfate. Environ. Eng. Sci. 29, 890–896 (2012)
Kmentova, H., Kment, S., Hubicka, Z., Remes, Z., Olejnicek, J., Cada, M., Krysa, J., Zboril, R.: Thermal sulfidation of α-Fe2O3 hematite to FeS2 pyrite thin electrodes: correlation between surface morphology and photoelectrochemical functionality. Catal. Today 313, 224–230 (2018)
Li, L., Liu, C., Qiu, Y., Mitsuzak, N., Chen, Z.: Convex-nanorods of α-Fe2O3/CQDs heterojunction photoanode synthesized by a facile hydrothermal method for highly efficient water oxidation. Int. J. Hydrog. Energy 42, 19654–19663 (2017)
Liu, Y., Yu, Y.X., Zhang, W.D.: Photoelectrochemical properties of Ni-doped Fe2O3 thin films prepared by electrodeposition. Electrochim. Acta 59, 121–127 (2012)
Ekwealor, A.B.C., Ezema, F.I.: Effects of precursor concentration on the optical and structural properties of Fe2O3 thin films synthesized in a polymer matrix by chemical bath deposition. J. Ovonic Res. 9, 35–43 (2013)
Li, Y., Li, H., Cao, R.: Facile fabrication of pure α-Fe2O3 nanoparticles via forced hydrolysis using microwave-assisted esterification and their sensing property. J. Am. Ceram. Soc. 92, 2188–2191 (2009)
Hu, X., Yu, J.C., Gong, J., Li, Q., Li, G.: α-Fe2O3 nanorings prepared by a microwave-assisted hydrothermal process and their sensing properties. Adv. Mater. 19, 2324–2329 (2007)
Liu, X., Chen, T., Chu, H., Niu, L., Sun, Z., Pan, L., Sun, C.Q.: Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 166, 12–16 (2015)
Youn, D.H., Jang, J.W., Kim, J.Y., Jang, J.S., Choi, S.H., Lee, J.S.: Fabrication of graphene-based electrode in less than a minute through hybrid microwave annealing. Sci. Rep. 4, 5492 (2014)
Hwang, J.Y., Shi, S., Xu, Z., Peterson, K.W.: Synthesis of monodispersed iron oxide particles by a large-scale microwave reactor. Chem. Eng. Commun. 193, 1586–1591 (2006)
Peiro, A.M., Ayllon, J.A., Peral, J., Domenech, X., Domingo, C.: Microwave activated chemical bath deposition (MW-CBD) of zinc oxide: influence of bath composition and substrate characteristics. J. Cryst. Growth 285, 6–16 (2005)
Mulmudi, H.K., Mathews, N., Dou, X.C., Xi, L.F., Pramana, S.S., Lam, Y.M., Mhaisalkar, S.G.: Controlled growth of hematite (α-Fe2O3) nanorod array on fluorine doped tin oxide: synthesis and photoelectrochemical properties. Electrochem. Commun. 13, 951–954 (2011)
Xiong, Q.Q., Tu, J.P., Ge, X., Wang, X.L., Gu, C.D.: One-step synthesis of hematite nanospindles from choline chloride/urea deep eutectic solvent with highly powerful storage versus lithium. J. Power Sources 274, 1–7 (2015)
Chirita, M., Banica, R., Ieta, A., Grozescu, I.: Fe-EDTA thermal decomposition, a route to highly crystalline hematite (Alpha Fe2O3) nanoparticle synthesis. Part. Sci. Technol. 28, 217–225 (2010)
Chen, M., Jiang, J., Zhou, X., Diao, G.: Preparation of akaganeite nanorods and their transformation to sphere shape hematite. J. Nanosci. Nanotechnol. 8, 3942–3948 (2008)
Kim, J., Choi, W.J.K., Choi, J., Hoffmann, M.R., Park, H.: Electrolysis of urea and urine for solar hydrogen. Catal. Today 199, 2–7 (2013)
Luan, J., Zheng, S., Hao, X., Luan, G., Wub, X., Zou, Z.: Photophysical and photocatalytic properties of novel M2BiNbO7 (M = In and Ga). J. Braz. Chem. Soc. 17, S1–S3 (2006)
Tauc, J., Grigorovic, R., Vancu, A.: Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 15, 627–637 (1966)
Wu, X.K., Huang, W.Q., Huang, Z.M., Qin, C.J., Dong, T.G., Wang, G., Tang, Y.L.: Fabrication of porous α-Fe2O3 nanoshuttles and their application for toluene sensors. Chin. Phys. B 26, 037302 (2017)
Lupan, O., Pauprte’, T., Chow, L., Viana, B., Pelle’, F., Ono, L.K., Cuenya, B.R., Heinrich, H.: Effects of annealing on properties of ZnO thin films prepared by electrochemical deposition in chloride medium. Appl. Surf. Sci. 256, 1895–1907 (2010)
Shinde, P.S., Choi, S.H., Kim, Y., Ryu, J., Jang, J.S.: Onset potential behavior in α-Fe2O3 photoanodes: the influence of surface and diffusion Sn doping on the surface states. Phys. Chem. Chem. Phys. 18, 2495–2509 (2016)
Kumari, S., Tripathi, C., Singh, A.P., Chauhan, D., Shrivastav, R., Dass, S., Satsangi, V.R.: Characterization of Zn-doped hematite thin films for photoelectrochemical splitting of water. Curr. Sci. 91, 1062–1064 (2006)
Lee, P.Y., Chang, S.P., Chang, S.J.: Photoelectrochemical characterization of n-type and p-type thin-film nanocrystalline Cu2ZnSnSe4 photocathodes. J. Environ. Chem. Eng. 3, 297–303 (2015)
Choi, H., Hong, Y., Ryu, H., Lee, W.: Photoelectrochemical properties of hematite thin films grown by MW-CBD. Surf. Coat. Technol. 333, 259–266 (2018)
Li, F., Li, J., Li, F., Gao, L., Long, X., Hu, Y., Wang, C., Wei, S., Jin, J., Ma, J.: Facile regrowth of Mg–Fe2O3/P–Fe2O3 homojunction photoelectrode for efficient solar water oxidation. J. Mater. Chem. A 6, 13412–13418 (2018)
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A3B01008959). This work was supported by the 2018 Inje University research Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hong, Y., Ryu, H. & Lee, WJ. Effects of Urea as an Additive in Fe2O3 Thin-Film Photoelectrodes. Electron. Mater. Lett. 15, 733–742 (2019). https://doi.org/10.1007/s13391-019-00174-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-019-00174-3