Abstract
Here we describe instrumental approaches for performing dual polarity ion confinement, transport, ion mobility separations, and reactions in structures for lossless ion manipulations (SLIM). Previous means of ion confinement in SLIM, based upon rf-generated pseudopotentials and DC fields for lateral confinement, cannot trap ions of opposite polarity simultaneously. Here we explore alternative approaches to provide simultaneous lateral confinement of both ion polarities. Traveling wave ion mobility (IM) separations experienced in such SLIM cause ions of both polarities to migrate in the same directions and exhibit similar separations. The ion motion (and relative motion of the two polarities) under both surfing and IM separation conditions are discussed. In surfing conditions the two polarities are transported losslessly and non-reactively in their respective potential minima (higher absolute voltage regions confine negative polarities, and lower absolute potential regions are populated by positive polarities). In separation mode, where ions roll over an overtaking traveling wave, the two polarities can interact during the rollovers. Strategies to minimize overlap of the two ion populations to prevent reactive losses during separations are presented. A theoretical treatment of the time scales over which two populations (injected into a DC field-free region of the dual polarity SLIM device) interact is considered, and SLIM designs for allowing ion/ion interactions and other manipulations with dual polarities at 4 Torr are presented.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical interactions of ions with other particles have been paired with MS since its infancy (e.g., formation of H3 +, N3 +, and HO2 + in Sir J. J. Thompson’s Mass Spectrograph were some of the first ion/molecule reactions observed [1]). There are also many recent and important biologically relevant applications of ion/molecule reactions [2], including reactions with post-translational modifications of peptides [3, 4], isomers of phosphorylated sugars [5], gas-phase hydrogen/deuterium exchange reactions [6, 7], and reactions of ozone with lipids [8]. The goal of these reactions is to reveal different aspects of ion structure and to quantify changes in structure that cannot be probed by the “traditional” mass spectrometric methods of measuring molecular and/or fragment ion masses.
Alternatively, reactions of oppositely charged ions for analytical MS have been pioneered by McLuckey and coworkers [9, 10]. Types of ion/ion reactions include charge transfer of protons from multiply protonated cations to anions [11], electron transfer [12], complex formation [13], and metal transfer [14]. Proton transfer reactions have been used for several applications, including simplification of a mixture of multiply charged ions (e.g., intact proteins [15] or peptides [16], as well as tandem MS fragments [17]), manipulation of charge states to yield higher sequence coverage [18], and conversion of many precursor protein charge states to a single charge state for much higher intensity of precursor ions than produced by electrospray [19, 20]. Electron transfer reactions led to the development of electron transfer dissociation [21], a widely applied fragmentation method complementary to collision induced dissociation, particularly important due to its ability to preserve post-translational modifications to peptides. Recently, ion/ion complex formation has been used for gas-phase covalent modifications [22]. The covalent reactions have included such examples as gas-phase Schiff base formation [22, 23], modification of amines with NHS-ester chemistry [24, 25], reactions with carboxylic acids [26, 27], and gas-phase oxidation of disulfide bonds [28, 29]. Ion/ion reactions are showing great promise in moving site-specific, traditionally solution-phase bioconjugation reactions into the gas phase.
The first cation to anion ion/ion proton transfer reactions studied showed that ion/ion reaction rates were dependent on the square of the protein charge state [11]. Since the anion was singly charged, this phenomenon is well explained by the rate constant (k, cm3/s) for ion/ion capture and the critical ion/ion separation in the orbiting ion complex formed (r, cm):
where v is the relative velocity (cm/s), Z1 and Z2 are the charge states of the cation and anion, e is the charge of the electron (electrostatic units), and μ is the reduced mass of the collision pair. With a large excess of anions, the rates proceeded as pseudo-first order. Therefore, a gas-phase chemistry environment with low relative velocities through, e.g., collisional dampening (higher pressures) and maximization of a field-free region, and the ability to trap large ion densities of reactants, is of interest to achieve the highest reaction rates. In addition, the ability to store the ion/ion reaction reagents and products, isolate products of interest, and pursue further steps of analysis, perhaps including fragmentation, more ion/ion or ion/molecule reactions, or even gas-phase separations, is desirable for integrating ion/ion reactions into a more holistic and complex gas-phase workflow, affording deeper analysis than currently available technologies.
Recent efforts in extended confinement [30, 31], ion manipulations [32], transporting ions [33,34,35], performing mobility based separations [35], and ion selection [31, 36] using structures for lossless ion manipulations (SLIM), have demonstrated this to provide a versatile platform to explore additional manipulations, including ion/ion reactions. Although demonstrations on SLIM so far have been confined to ions of a single polarity (predominantly cations), the ability to perform ion/ion reactions is possible with designs that allow dual polarity transport, storage, and separations. To that end, we have begun explorations including ion trajectory modeling in SLIM modules to accommodate dual polarities losslessly and also explore the possibility of ion/ion reactions. Ion/ion reactions have already been performed with traveling wave IM devices for proton transfer and electron transfer dissociation, and separation and characterization of reaction products [37,38,39]. Developing and expanding on these capabilities with the flexibility of the SLIM device will allow for a much greater number of more complex series of reactions, mobility separations, and other manipulations. The following illustrates initial SLIM module designs for the confinement, transport, and mutual storage of ions of opposing polarities.
Methods
Ion trajectory simulations were performed using SIMION 8.1 (Scientific Instrument Services, Ringoes, NJ, USA). The SLIM geometries for testing dual polarity confinement were built using the SIMION graphical user interface or importing models from CAD software (SolidWorks, Dassault Systems Solidworks Corporation, Waltham, MA, USA). The potential arrays were generated by using the SIMION refine feature with a convergence criterion of 0.001. The potential arrays created included 10 distinct electrode potentials. Two electrodes had rf voltages (~300 Vp-p amplitude) applied to them. The other eight electrode potentials had time varying DC profiles (i.e. TW). The time varying voltages were defined on each electrode using an in-house Lua programming code compatible with SIMION. The specific voltages applied to the different rf phases and TW electrodes and their layouts are discussed later in the manuscript. The collisions between ions and neutrals in the trajectory simulations used the SDS model [32, 40,41,42]. The ions were injected into the SLIM domain with an initial kinetic energy of zero. Since any motion of ions is quickly (within a few nanoseconds) thermalized at a pressure of 4 Torr, the initial KE has no significant effect on the trajectories: the ion velocity is only dependent on the instantaneous electric field. Also, ion potentials were calculated using an open source solver (OpenFoam 4.0) [43] (which is a generic partial differential equation solver), by solving the Laplace equation. The Laplace solution was computed by using a finite volume discretization of the SLIM domain and by using the appropriate potentials applied to the electrodes [44].
Results and Discussion
The confinement of oppositely charged ions inside SLIM requires modification of previous SLIM designs. In previous SLIM designs, rf-generated pseudopotentials are used to confine ions away from the two parallel surfaces [33, 34, 45] and lateral confinement is provided by use of DC ‘guard’ electrodes. In this scheme of single polarity confinement and transport, guard electrodes with a slightly higher dc potential (up to +5 V) than the average TW DC potential provide effective lateral confinement for ions with positive polarity. However, the combined effective potential and guard fields created within SLIM are not suitable for confining opposite polarity ions at the same time. Figure 1a shows the confinement pseudo-potentials inside the traditional SLIM-TW design [34] using 300 Vp-p rf, 30 Vp-p for TW, and a 20 V guard voltage (i.e., +5 V bias). Figure 1 top panel shows a SLIM-TW configuration with the ion conduits to confine ions for a positively charged species (m/z 622, z = 1, K0 = 1.17 cm2/Vs). A ‘dumbbell’ shaped confinement region is evident. For the positive polarity, the effective potential provides confinement in the center. For the negative polarity (which migrate to higher voltage regions) there is opportunity for the ions to be lost laterally between the SLIM surfaces. Ion trajectory simulations performed using SIMION also show the confinement of positively charged ions (Figure 1b). While the rf is sufficient to prevent ion losses to those electrode regions, the positive potential of the guard creates an attractive potential for the negatively charged species and results in immediate ion losses. Similarly, when the guard bias is changed to –5 V, negatively charged ions are confined but positively charged ions are lost. Thus, ion conduits that can confine both positive and negative ions simultaneously require an alternate means to confine ions in the lateral direction.
(a) Schematic of SLIM-TW board layout, showing ion conduits created in SLIM for confinement of positive ions (b) Top panel shows the ion trajectories for positive ions (red traces) and negative ions (black traces) under the same effective potential conditions. Bottom panel shows the ion trajectories when the guard potential is made negative (–5 V), in which case the positive ions are lost laterally while negative ions are confined
Owing to the flexibility of SLIM, relatively complex electrode structures and voltages can be patterned on the surfaces opening alternatives for lateral confinement. While DC-only potentials are insufficient to confine dual polarities, in this work we develop alternative approaches for applying dynamic potentials in SLIM (i.e., rf) to effect lateral confinement. The use of only rf electrodes for confinement away from surfaces and also for lateral confinement, meant that a potential wall needed to be created between the two SLIM surfaces to restrict lateral ion motion. Thus, unlike in previous SLIM designs for single polarities (where two boards were arranged parallel to each other), in the new design an “anti-parallel” arrangement of electrodes was used. A configuration of four rf and three traveling wave electrodes (referred to as 4,3 SLIM configuration) for the new module designs is shown in Figure 2a. While an electrode arrangement has alternate rf phases with interleaved TW electrodes, the other SLIM surface has corresponding rf electrodes carrying the opposite phase of rf. The TW electrodes are similarly interleaved (see Figure 2a and compare with Figure 1a). The opposite polarity of any two adjacent rf electrodes provides confinement away from the surfaces. The opposite polarity of rf on the other board surface provides the confinement potential preventing the ions from escaping laterally between the boards. A 4,3 SLIM design [46] is shown in Figure 2. This is easily extendable to more rf electrodes to accommodate a greater number of ions. One of the significant advantages of this SLIM arrangement is that the DC guard electrodes are eliminated. This allows for serpentine path SLIM [47] to be more efficient; the increased lateral width available by removing the DC guards can allow more U-turns to be accommodated in a given region. The TW electrodes for ion motion are interleaved as we have done in previous TW SLIM. Figure 2a right panel shows the layout of the two boards with line-of-sight looking in the direction of ion motion between the boards. The confinement of ions is shown by the SIMION ion trajectories superimposed. Figure 2b shows the effective ion conduits created by the setup for both positive and negative ions. The rf frequency in both cases is 1 MHz and rf amplitude is 350 Vp-p. The shape of the confinement region is more quadrupolar than the previous TW-SLIM designs (as seen from the Figure 1a and b). The bottom panel of Figure 2b shows the effective potential along lateral axis (along the Y axis) between the two boards. The potential well is clearly quadrupolar for both positive and negative ions, unlike in the case of the TW-SLIM with DC guards where the negative polarity ions experience no lateral confinement (Figure 1b). A total charge of \( Q\le {\in}_o{\displaystyle \oint \overrightarrow{E}.\overrightarrow{d} A}. \), where \( {\displaystyle \oint \overrightarrow{E}.\overrightarrow{d} A}. \) is the surface integral of the electric field (e.g. along an iso-surface of the effective potential value of 20 V as shown in Figure 2b) will remain confined within the ion conduit in the presence of traveling waves. Iso-surfaces less than 20 V will confine a smaller number of ions. The size of the ion conduit within which ions will be confined depends on the total charge being carried by the SLIM device and the details related to the moving voltage profile of the TW. Figure 2c shows the SIMION simulation of ion trajectories of positive and negative polarities of an ion with m/z 622 and reduced mobility Ko = 1.17 cm2/Vs. The ability to confine and transport ions of dual polarities enables building devices to perform ion mobility-based separations for positive and negative ions simultaneously. The use of traveling waves symmetric about 0 V (±20 V or 40 Vp-p) with rf guards allows ions to be confined and transported. The arrival time distributions (ATDs) and the peak resolutions (for TW-SLIM path length of 4 cm) obtained for ions with m/z 622 and m/z 922 with positive charges and hypothetical ions of same mass and mobility but with a negative charge are shown in Figure 2d. The ion trajectory simulations are also shown. Each species had 500 ions. The ions traverse a simulated path of 15 cm. The ATDs demonstrate similar performance for both polarities, thus confirming the ability to use the SLIM module design for IM separations for both the polarities. Any slight differences in the arrival time distributions arise due to the fact that at the initial condition of the simulation, both polarities see the same phase of the TW, which means one of the polarities begins with a backward rollover, whereas the other polarity begins with a forward motion. These initial effects become negligible for long path length separations.
(a) Left panel shows the schematic of the board design to confine dual polarities. Guard electrodes as shown in purple in Figure 1a are replaced with rf electrodes and the boards are paired in such a way that counter-polarity rf electrodes are laid out in the opposing board. Right panel shows the view between the boards in the YZ plane with SIMION ion trajectories super-imposed showing quadrupole fields confining the ions. (b) Top panel shows ion conduits created for confining ions; bottom panel shows the potential on an equidistant line between the SLIM surfaces; (c) ion trajectory simulations in SIMION showing lossless transmission of both positive (red traces) and negative (black traces) ions. (d) Arrival time distributions (ATDs) for m/z 622 (Ko 1.17 cm2/Vs) and m/z 922 (Ko = 0.97 cm2/Vs) over a path length of 4 cm
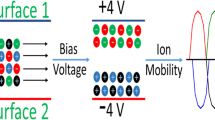
SLIM modules that are suitable for performing dual polarity ion motion and separations require performing separations without ion/ion interactions unless desired. The reactions can take place by either ion/ion recombination leading to loss of signal for either the species or charge state alteration. Figure 3a shows the distance between two ions (of same mobility and opposite polarity, m/z 622, Ko = 1.17 cm2/Vs) as they traverse along the length of the device in the surfing mode [48]. The surfing mode of operating a TW device is typically achieved at very low traveling wave speeds (i.e., when speed of the wave is lower than then instantaneous ion velocity [34], typically < ~50 m/s for Ko = 1 cm2/Vs at 4 Torr), and in this mode ions are confined within the TW troughs [47]. Since the ions with opposite polarities have potential minima at opposite ends of the potential profile, the surfing mode ensures effective spatial segregation between the two species while they traverse at the same speed, as seen in Figure 3a. As the ions of two polarities travel through the device they populate their respective potential minima and remain confined within these regions. The width of the potential minima were about four electrodes wide (as four consecutive electrodes have high voltage and the remaining have low voltage as shown in in the inset of Figure 3a) [34]. Since in this simulation, the length of each electrode was 1 mm, the two ions (which start at the same point initially in the simulation) are separated by a distance of ~4 mm. However, in the separation mode, ions are passing through other potential minima/troughs, as they “roll over” the passing wave profile. Thus, there will be instances when the two polarities pass each other. Figure 3b shows the distance between two ions of same mobility but opposite polarity varies between 0 mm (when they pass each other) and ~4 mm. The relative velocity between the ions (Figure 3b inset) varies between 150 m/s and –150 m/s. Ion/ion recombination for these two singly-charged ions can occur if the distance between the opposite polarities is lower than a critical value (rcritical), which can be calculated by considering the magnitudes of the relative velocity. At a relative velocity of 2 m/s, the critical radius was 0.162 mm (see discussion below), whereas at relative velocity higher than 6 m/s the critical distance needed for reaction was less than a few microns (thus reducing the probability of reaction). However at relative velocities lower than 2 m/s, the ion/ion recombination rate can be very high. The relative velocity of the ions was found to be less than 3 m/s magnitude for ~2.5% of the total flight time. Thus, while they are separating, it can be expected that the ions have a small but finite possibility of interaction, and when the separation time is long enough, ion/ion reactions can occur. The reactions can be mitigated by biasing the voltages on rf guards to laterally separate the two polarities (as shown in Figure 3c). The plumes colored red and black represent negative and positive polarities of ions and the intensity of color corresponds to the relative number of ions. Above 2 V bias, the two plumes are fully separated (Figure 3c and inset). At 1 V bias, ~25% of the plumes overlap. Additionally, biasing the two surfaces to different DC voltages (+7 V on one board and 0 V on the other one in this case shown in Figure 3d) is another way to segregate the populations and prevent ion/ion reactions while performing separations simultaneously for both polarities. This allows simultaneous dual polarity IMS separations. Dynamically controlling these voltages allows controlled ion population overlap; an ion/ion rate reduction technique that could eventually be utilized as a SLIM analog to ion parallel parking (where reaction rates are slowed over a broader m/z range) [49, 50].
(a) The evolution of distance between two hypothetical ions of same mass, mobility, and opposite charge as they traverse through the dual polarity SLIM device in the surfing mode where ions are confined to bins of TW. Ions are started at same point in the simulation and they separate into respective potential minima as shown in the inset, where positive ions (blue) populate low potential regions shown by (darker colored TW electrodes) and negative ions (red) populate the high voltage regions. (b) Evolution of distance between polarities when they are in the separation mode. Ions of same mobility and opposite polarity are considered, showing that they pass each other during every cycle of the TW. The distance between polarities is lesser than critical distance for ion/ion reaction for a small fraction of time (discussion in text). Inset shows the relative velocities between the ions (magnitude varying between 1 m/s and 150 m/s) over the course of their transport. (c) The two polarities are laterally separated to by applying +4 V and –4 V guard biases on each side. Inset shows separation between the plumes at 1 V (with ~25% overlap of the plumes) and 2 V biases. (d) The two polarities separated between the two surfaces by biasing one surface at +7 V
Although ion trajectory simulations can predict the extent of spatial overlap of ions of both charges and relative velocities of ions of each polarity, they do not consider space charge or the effects of ions of opposite polarities upon each other. Therefore, to determine the efficacy of ion/ion reactions, a theoretical treatment of a model reaction is useful. In this example, we use the triply protonated peptide neurotensin (m/z 558.65 and reduced mobility 1.2 cm2/Vs) and deprotonated 1H,1H-perfluoro-1-octanol (m/z 399.07 and estimated reduced mobility 0.9 cm2/Vs) as the reactants, assuming a large excess of the anionic reagent, with proton transfer from neurotensin. The schematic for a device to allow such ion/ion interactions is shown in Figure 4a. Positive and negative polarities are introduced from opposite directions (as shown in Figure 4a) to prevent any potential reactions during transport. However, alternative designs can be envisioned where the two polarities are introduced from the same direction in a time-dependent manner. A central field-free region with 8 TW electrodes at 0 V is created for ion/ion interaction. Once in the field-free region, the thermal velocity attributable to Brownian motion of the ions and the attractive potential between the two polarities determine the rate at which they approach each other. To estimate the possibility of ion/ion reactions, we treat an ensemble of ions of each charge as a point charge with a total amount of charge Q in Coulombs providing an attractive potential over a long distance upon another ensemble of ions of opposite charge. The long range attractive potential created by a cation of charge state zp is given by
where U p is the attractive potential, Q p is the total positive charge in Coulombs, N p is the number of positive charges, z p is the charge state, e is the elementary charge, and r is the distance. Note that in the above expression, we calculated an ensemble of ions as a point change with a total charge given by the product of the number of charges and the value of elementary charge (which directly follows from Gauss’s law [51]). The force experienced by the two polarities due to their mutual attractive potentials will be:
where F is the force of Coulombic attraction, Q n is the total negative charge, N n is the total number of negatively charged ions, z n is the charge state of the negative ion, and r is the distance between the two ions. Since the ions are confined at pressures at a few torr, at these low E/N conditions, ions move according to their mobilities. Thus, the relative velocity v(r) between the ions becomes:
where K p and K n are the ion mobilities of the positively and negatively charged ions, respectively. The ion clouds proceed to attract each other in the field-free region and form the ion/ion reaction complex. The time frame over which the ion populations would approach each other depends on their initial distance apart (16 mm here, corresponding to the length of the field-free SLIM region shown in Figure 4a) and their relative velocity (governed by Equation 4).
(a) SLIM design with the two polarities introduced from either side of a 16 mm long DC field free region. (b) The relative approach velocity between populations (of 100,000 charges each of protonated peptide neurotensin m/z 558.6473, and reduced mobility 0.9 cm2/Vs and deprotonated 1H,1H-Perfluoro-1-octanol m/z 399.07 and reduced mobility 1.2 cm2/Vs) as a function distance between polarities. (c) Distance between the two polarities as a function of time beginning with an initial separation of ~14 mm
As shown in Figure 4a, the two populations injected from two sides of a field-free region span ~16 mm. Due to the abundance of collisions with the background gas, the relative velocity of the ions is low enough to form the bound orbit complexes once the ions are close enough. Thus, applying Equation 1, the critical separation for bound orbit formation is 162 μm. A relative velocity of 2 m/s was used for this calculation, and this conforms to the relative velocity that the ions experience as they approach each other (at a low relative velocity of ~<1 m/s as shown in Figure 4b). Figure 4c also shows the time taken for the progressive reduction in the distance between the two ionic species. In the simulation (Figure 4a), the injected populations diffuse over the region (of ~15 mm) in about ~200 ms based on a \( \sqrt{2 Dt} \) estimate. As shown in Figure 4c, the presence of attractive forces significantly enhances the rate at which the two polarities would approach each other (it takes 90 ms for 100,000 cations and 100,000 anions to reach an approach distance of ~0.1 mm at 4 Torr as shown in Figure 4c). This rate of approach of the two polarities would be higher at lower pressures. In practice, a large excess of the reagent population should be used to maximize ion/ion reaction rates, with pseudo-first order reaction rates. In addition, traveling waves can be used intermittently to decrease the size of the mutual storage region for better spatial overlap. However, care must be used with this approach in order to maintain low relative velocities. After the reaction, the products can then be transported to the detector in the surfing mode (to isolate products of both polarities and prevent further reactions) or into additional regions, e.g., reactions or mobility separations.
Conclusions
The basis for novel SLIM designs capable of simultaneously manipulating, separating, and transmitting two ion polarities is presented. Previous SLIM modules have utilized DC fields for lateral confinement but cannot confine both polarities of ions simultaneously. A design that utilizes only rf fields for lateral confinement is presented, with efficient ion confinement and transmission of ions of both polarities. It was shown that at very low traveling wave speeds, in the so-called “surfing” mode, oppositely charged ions are separated in their own “traveling traps” that effectively prevent ion/ion reactions from occurring. However, with faster TW speeds (i.e., separation conditions), there is a possibility for ion/ion interactions due to the movement of ions between the traveling traps. This process can be mitigated by the application of a DC bias across the guard electrodes or offsetting the DC bias on one SLIM surface against the other. The ability to confine, transport, and separate simultaneously dual polarities of ions without losses helps perform IMS analyses with high sensitivity and selectivity. To be able to inject and transport both the polarities at the same time without any interaction also helps perform very long path length separations at a higher duty cycle compared to separate injections used to perform separations on two polarities. It will also allow for the physical effects of charge/charge attraction to be investigated between oppositely charged ions. In addition, the possibilities for conducting ion/ion reactions inside a SLIM module were examined with a combination of trajectory simulations and a point-charge treatment of ion/ion capture. Utilizing a DC field-free region of the dual polarity SLIM electrode geometry, reactions are predicted to proceed with very high rate constants due to rapid collisional dampening at 4 Torr pressures. We will employ the design to begin experimental exploration of ion/ion reactions in SLIM.
References
Thomson, J.J.: Rays of positive electricity and their application to chemical analyses. Longmans, Green, and Co., London (1913)
Osburn, S., Ryzhov, V.: Ion–molecule reactions: analytical and structural tool. Anal. Chem. 85, 769–778 (2013)
Gronert, S., Li, K.H., Horiuchi, M.: Manipulating the fragmentation patterns of phosphopeptides via gas-phase boron derivatization: determining phosphorylation sites in peptides with multiple serines. J. Am. Soc. Mass Spectrom. 16, 1905–1914 (2005)
Osburn, S., O'Hair, R.A.J., Black, S.M., Ryzhov, V.: Post-translational modification in the gas phase: mechanism of cysteine S-nitrosylation via ion–molecule reactions. Rapid Commun. Mass Spectrom. 25, 3216–3222 (2011)
Gao, H., Petzold, C.J., Leavell, M.D., Leary, J.A.: Investigation of ion/molecule reactions as a quantification method for phosphorylated positional isomers: an FT-ICR approach. J. Am. Soc. Mass Spectrom. 14, 916–924 (2003)
Kang, Y., Terrier, P., Ding, C.F., Douglas, D.J.: Solution and gas-phase H/D exchange of protein–small molecule complexes: Cex and its inhibitors. J. Am. Soc. Mass Spectrom. 23, 57–67 (2012)
Wright, P.J., Douglas, D.J.: Gas-phase H/D exchange and collision cross sections of hemoglobin monomers, dimers, and tetramers. J. Am. Soc. Mass Spectrom. 20, 484–495 (2009)
Brown, S.H.J., Mitchell, T.W., Blanksby, S.J.: Analysis of unsaturated lipids by ozone-induced dissociation. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 1811, 807–817 (2011)
Prentice, B.M., McLuckey, S.A.: Gas-phase ion/ion reactions of peptides and proteins: acid/base, redox, and covalent chemistries. Chem. Commun. 49, 947–965 (2013)
Huang, T.Y., McLuckey, S.A.: Gas-phase chemistry of multiply charged bioions in analytical mass spectrometry. In: Yeung, E.S., Zare, R.N. (eds.) Annu. Rev. Palo Alto, CA (2010)
Stephenson, J.L., McLuckey, S.A.: Ion/ion reactions in the gas phase: proton transfer reactions involving multiply-charged proteins. J. Am. Chem. Soc. 118, 7390–7397 (1996)
Stephenson, J.L., McLuckey, S.A.: Charge reduction of oligonucleotide anions via gas-phase electron transfer to xenon cations. Rapid Commun. Mass Spectrom. 11, 875–880 (1997)
Wells, J.M., Chrisman, P.A., McLuckey, S.A.: Formation and characterization of protein−protein complexes in vacuo. J. Am. Chem. Soc. 125, 7238–7249 (2003)
Newton, K.A., Amunugama, R., McLuckey, S.A.: Gas-phase ion/ion reactions of multiply protonated polypeptides with metal containing anions. J. Phys. Chem. A 109, 3608–3616 (2005)
Xia, Y., Chrisman, P.A., Erickson, D.E., Liu, J., Liang, X., Londry, F.A., Yang, M.J., McLuckey, S.A.: Implementation of ion/ion reactions in a quadrupole/time-of-flight tandem mass spectrometer. Anal. Chem. 78, 4146–4154 (2006)
Stephenson, J.L., McLuckey, S.A.: Ion/ion reactions for oligopeptide mixture analysis: application to mixtures comprised of 0.5–100 kDa components. J. Am. Soc. Mass Spectrom. 9, 585–596 (1998)
Stephenson, J.L., McLuckey, S.A.: Simplification of product ion spectra derived from multiply charged parent ions via ion/ion chemistry. Anal. Chem. 70, 3533–3544 (1998)
Hogan, J.M., McLuckey, S.A.: Charge state-dependent collision-induced dissociation of native and reduced porcine elastase. J. Mass Spectrom. 38, 245–256 (2003)
McLuckey, S.A., Reid, G.E., Wells, J.M.: Ion parking during ion/ion reactions in electrodynamic ion traps. Anal. Chem. 74, 336–346 (2002)
He, M., Reid, G.E., Shang, H., Lee, G.U., McLuckey, S.A.: Dissociation of multiple protein ion charge states following a single gas-phase purification and concentration procedure. Anal. Chem. 74, 4653–4661 (2002)
Syka, J.E.P., Coon, J.J., Schroeder, M.J., Shabanowitz, J., Hunt, D.F.: Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 101, 9528–9533 (2004)
Han, H., McLuckey, S.A.: Selective covalent bond formation in polypeptide ions via gas-phase ion/ion reaction chemistry. J. Am. Chem. Soc. 131, 12884–12885 (2009)
Stutzman, J.R., Luongo, C.A., McLuckey, S.A.: Covalent and noncovalent binding in the ion/ion charge inversion of peptide cations with benzene-disulfonic acid anions. J. Mass Spectrom. 47, 669–675 (2012)
Mentinova, M., McLuckey, S.A.: Covalent modification of gaseous peptide ions with N-hydroxysuccinimide ester reagent ions. J. Am. Chem. Soc. 132, 18248–18257 (2010)
Webb, I.K., Mentinova, M., Mcgee, W.M., McLuckey, S.A.: Gas-phase intramolecular protein crosslinking via ion/ion reactions: ubiquitin and a homobifunctional sulfo-NHS ester. J. Am. Soc. Mass Spectrom. 24, 733–743 (2013)
Prentice, B.M., Gilbert, J.D., Stutzman, J.R., Forrest, W.P., McLuckey, S.A.: Gas-phase reactivity of carboxylic acid functional groups with carbodiimides. J. Am. Soc. Mass Spectrom. 24, 30–37 (2013)
Peng, Z., McGee, W.M., Bu, J., Barefoot, N.Z., McLuckey, S.A.: Gas phase reactivity of carboxylates with N-hydroxysuccinimide esters. J. Am. Soc. Mass Spectrom. 26, 174–180 (2015)
Pilo, A.L., McLuckey, S.A.: Oxidation of methionine residues in polypeptide ions via gas-phase ion/ion chemistry. J. Am. Soc. Mass Spectrom. 25, 1049–1057 (2014)
Pilo, A.L., McLuckey, S.A.: Selective gas-phase ion/ion reactions: enabling disulfide mapping via oxidation and cleavage of disulfide bonds in intermolecularly-linked polypeptide ions. Anal. Chem. 88, 8972–8979 (2016)
Zhang, X., Garimella, S.V., Prost, S.A., Webb, I.K., Chen, T.C., Tang, K., Tolmachev, A.V., Norheim, R.V., Baker, E.S., Anderson, G.A., Ibrahim, Y.M., Smith, R.D.: Ion trapping, storage, and ejection in structures for lossless ion manipulations. Anal. Chem. 87, 6010–6016 (2015)
Chen, T.C., Ibrahim, Y.M., Webb, I.K., Garimella, S.V., Zhang, X., Hamid, A.M., Deng, L., Karnesky, W.E., Prost, S.A., Sandoval, J.A., Norheim, R.V., Anderson, G.A., Tolmachev, A.V., Baker, E.S., Smith, R.D.: Mobility-selected ion trapping and enrichment using structures for lossless ion manipulations. Anal. Chem. 88, 1728–1733 (2016)
Garimella, S.V.B., Ibrahim, Y.M., Webb, I.K., Ipsen, A.B., Chen, T.C., Tolmachev, A.V., Baker, E.S., Anderson, G.A., Smith, R.D.: Ion manipulations in structures for lossless ion manipulations (SLIM): computational evaluation of a 90° turn and a switch. Analyst 14, 6845–6852 (2015)
Webb, I.K., Garimella, S.V.B., Tolmachev, A.V., Chen, T.C., Zhang, X.Y., Norheim, R.V., Prost, S.A., LaMarche, B., Anderson, G.A., Ibrahim, Y.M., Smith, R.D.: Experimental evaluation and optimization of structures for lossless ion manipulations for ion mobility spectrometry with time-of-flight mass spectrometry. Anal. Chem. 86, 9169–9176 (2014)
Hamid, A.M., Ibrahim, Y.M., Garimella, S.V.B., Webb, I.K., Deng, L.L., Chen, T.C., Anderson, G.A., Prost, S.A., Norheim, R.V., Tolmachev, A.V., Smith, R.D.: Characterization of traveling wave ion mobility separations in structures for lossless ion manipulations. Anal. Chem. 87, 11301–11308 (2015)
Deng, L., Ibrahim, Y.M., Hamid, A.M., Garimella, S.V., Webb, I.K., Zheng, X., Prost, S.A., Sandoval, J.A., Norheim, R.V., Anderson, G.A., Tolmachev, A.V., Baker, E.S., Smith, R.D.: Ultra-high resolution ion mobility separations utilizing traveling waves in a 13-m serpentine path length structures for lossless ion manipulations module. Anal. Chem. 89, 4628–4634 (2016)
Webb, I.K., Garimella, S.V., Tolmachev, A.V., Chen, T.C., Zhang, X., Cox, J.T., Norheim, R.V., Prost, S.A., LaMarche, B., Anderson, G.A., Ibrahim, Y.M., Smith, R.D.: Mobility-resolved ion selection in uniform drift field ion mobility spectrometry/mass spectrometry: dynamic switching in structures for lossless ion manipulations. Anal. Chem. 86, 9632–9637 (2014)
Williams, J.P., Brown, J.M., Campuzano, I., Sadler, P.J.: Identifying drug metallation sites on peptides using electron transfer dissociation (ETD), collision induced dissociation (CID), and ion mobility-mass spectrometry (IM-MS). Chem. Commun. 46, 5458–5460 (2010)
Rand, K.D., Pringle, S.D., Morris, M., Engen, J.R., Brown, J.M.: ETD in a traveling wave ion guide at tuned Z-spray ion source conditions allows for site-specific hydrogen/deuterium exchange measurements. J. Am. Soc. Mass Spectrom. 22, 1784 (2011)
Laszlo, K.J., Bush, M.F.: Analysis of native-like proteins and protein complexes using cation to anion proton transfer reactions (CAPTR). J. Am. Soc. Mass Spectrom. 26, 2152–2161 (2015)
Garimella, S.V.B., Ibrahim, Y.M., Tang, K.Q., Webb, I.K., Baker, E.S., Tolmachev, A.V., Chen, T.C., Anderson, G.A., Smith, R.D.: Spatial ion peak compression and its utility in ion mobility spectrometry. J. Am. Soc. Mass Spectrom. 27, 1128–1135 (2016)
Appelhans, A.D., Dahl, D.A.: SIMION ion optics simulations at atmospheric pressure. Int. J. Mass Spectrom. 244, 1–14 (2005)
Lai, H., McJunkin, T.R., Miller, C.J., Scott, J.R., Almirall, J.R.: The predictive power of SIMION/SDS simulation software for modeling ion mobility spectrometry instruments. Int. J. Mass Spectrom. 276, 1–8 (2008)
Weller, H.G., Tabor, G., Jasak, H., Fureby, C.: A tensorial approach to computational continuum mechanics using object-oriented techniques. Comput. Phys. 12, 620–631 (1998)
Patankar, S.V.: In: Minkowycz, W.J., Sparrow, E.M. (eds.) Numerical Heat Transfer and Fluid Flow. McGraw-Hill, Washington (1980)
Garimella, S.V.B., Ibrahim, Y.M., Webb, I.K., Tolmachev, A.V., Zhang, X.Y., Prost, S.A., Anderson, G.A., Smith, R.D.: Simulation of electric potentials and ion motion in planar electrode structures for lossless ion manipulations (SLIM). J. Am. Soc. Mass Spectrom. 25, 1890–1896 (2014)
Wojcik, R., Webb, I.K., Deng, L., Garimella, S.V.B., Prost, S.A., Ibrahim, Y.M., Baker, E.S., Smith, R.D.: Lipid and glycolipid isomer analyses using ultra-high resolution ion mobility spectrometry separations. Int. J. Mol. Sci. 18. doi:10.3390/ijms18010183 (2017)
Garimella, S.V.B., Hamid, A.M., Deng, L., Ibrahim, Y.M., Webb, I.K., Baker, E.S., Prost, S.A., Norheim, R.V., Anderson, G.A., Smith, R.D.: Squeezing of ion populations and peaks in traveling wave ion mobility separations and structures for lossless ion manipulations using compression ratio ion mobility programming. Anal. Chem. 88, 11877–11885 (2016)
Giles, K., Williams, J.P., Campuzano, I.: Enhancements in traveling wave ion mobility resolution. Rapid Commun. Mass Spectrom. 25, 1559–1566 (2011)
Chrisman, P.A., Pitteri, S.J., McLuckey, S.A.: Parallel ion parking: improving conversion of parents to first-generation products in electron transfer dissociation. Anal. Chem. 77, 3411–3414 (2005)
Chrisman, P.A., Pitteri, S.J., McLuckey, S.A.: Parallel ion parking of protein mixtures. Anal. Chem. 78, 310–316 (2006)
Halliday, D., Resnick, R.: Fundamentals of physics. Wiley, New York (1970)
Acknowledgments
Portions of this research were supported by the Department of Energy Office of Biological and Environmental Research Program under the Pan-Omics Program and by the National Institute of General Medical Sciences (P41 GM103493). Work was performed in the Environmental Molecular Sciences Laboratory (EMSL), a DOE national scientific user facility at the Pacific Northwest National Laboratory (PNNL) in Richland, WA. PNNL is operated by Battelle for the DOE under Contract DE-AC05-76RL0 1830.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sandilya V. B. Garimella and Ian K. Webb contributed equally to this work.
Rights and permissions
About this article
Cite this article
Garimella, S.V.B., Webb, I.K., Prabhakaran, A. et al. Design of a TW-SLIM Module for Dual Polarity Confinement, Transport, and Reactions. J. Am. Soc. Mass Spectrom. 28, 1442–1449 (2017). https://doi.org/10.1007/s13361-017-1680-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1680-5