Abstract
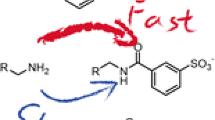
N-hydroxysuccinimide (NHS) esters have been used for gas-phase conjugation reactions with peptides at nucleophilic sites, such as primary amines (N-terminus, ε-amine of lysine) or guanidines, by forming amide bonds through a nucleophilic attack on the carbonyl carbon. The carboxylate has recently been found to also be a reactive nucleophile capable of initiating a similar nucleophilic attack to form a labile anhydride bond. The fragile bond is easily cleaved, resulting in an oxygen transfer from the carboxylate-containing species to the reagent, nominally observed as a water transfer. This reactivity is shown for both peptides and non-peptidic species. Reagents isotopically labeled with O18 were used to confirm reactivity. This constitutes an example of distinct differences in reactivity of carboxylates between the gas phase, where they are shown to be reactive, and the solution phase, where they are not regarded as reactive with NHS esters.

ᅟ







Similar content being viewed by others
References
Yang, W.C., Mirzaei, H., Liu, X.P., Regnier, F.E.: Enhancement of amino acid detection and quantification by electrospray ionization mass spectromety. Anal. Chem. 78, 4702–4708 (2006)
Gygi, S.P., Rist, B., Gerber, S.A., Turecek, F., Gelb, M.H., Aebersold, R.: Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 17, 994–999 (1999)
Ross, P.L., Huang, Y.N., Marchese, J.N., Williamson, B., Parker, K., Hattan, S., Khainovski, N., Pillai, S., Dey, S., Daniels, S., Purkayastha, S., Juhasz, P., Martin, S., Bartlet-Jones, M., He, F., Jacobson, A., Pappin, D.J.: Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3, 1154–1169 (2004)
Beardsley, R.L., Sharon, L.A., Reilly, J.P.: Peptide de novo sequencing facilitated by a dual-labeling strategy. Anal. Chem. 77, 6300–6309 (2005)
Madsen, J.A., Brodbelt, J.S.: Simplifying fragmentation patterns of multiply charged peptides by N-terminal derivatization and electron transfer collision activated dissociation. Anal. Chem. 81, 3645–3653 (2009)
Mendoza, V.L., Vachet, R.W.: Simplifying fragmentation patterns of multiply charged peptides by N-terminal derivatization and electron transfer collision activated dissociation. Mass Spectrom. Rev. 28, 785–815 (2009)
Gao, Y., McLuckey, S.A.: Electron transfer followed by collision-induced dissociation (NET-CID) for generating sequence information from backbone modified oligonucleotide anions. Rapid Commun. Mass Spectrom. 27, 249–257 (2013)
Gao, G., Yang, J., Cancilla, M.T., Meng, F., McLuckey, S.A.: Top-down interrogation of chemically modified oligonucleotides by negative electron transfer and collision induced dissociation. Anal. Chem. 85, 4713–4720 (2013)
Huang, T.-Y., McLuckey, S.A.: Gas-phase chemistry of multiply-charged bio-ions in analytical mass spectrometry. Annu. Rev. Anal. Chem. 3, 365–385 (2010)
Mentinova, M., McLuckey, S.A.: Covalent modification of gaseous peptide ions with N-hydroxysuccinimide ester reagent ions. J. Am. Chem. Soc. 132, 18248–18257 (2010)
Mentinova, M., McLuckey, S.A.: Intra- and inter-molecular cross-linking of peptide ions in the gas phase: reagents and conditions. J. Am. Soc. Mass Spectrom. 22, 912–921 (2011)
Webb, I.K., Mentinova, M., McGee, W.M., McLuckey, S.A.: Gas-phase intramolecular protein crosslinking via ion/ion reactions: ubiquitin and a homobifunctional sulfo-NHS Ester. J. Am. Soc. Mass Spectrom. 24, 733–743 (2013)
Han, H., McLuckey, S.A.: Selective covalent bond formation in polypeptide ions via gas-phase ion/ion reaction chemistry. J. Am. Chem. Soc. 131, 12884–12885 (2009)
Hassell, K.M., Stutzman, J.R., McLuckey, S.A.: Gas-phase bioconjugation of peptides via ion/ion charge inversion: Schiff base formation on the conversion of cations to anions. Anal. Chem. 82, 1594–1597 (2010)
Stutzman, J.R., Hassell, K.M., McLuckey, S.A.: Dissociation behavior of tryptic and intramolecular disulfide-linked peptide ions modified in the gas phase via ion/ion reactions. Int. J. Mass Spectrom. 312, 195–200 (2012)
Mentinova, M., Barefoot, N.Z., McLuckey, S.A.: Solution versus gas-phase modification of peptide cations with NHS-ester reagents. J. Am. Soc. Mass Spectrom. 23, 282–289 (2011)
McGee, W.M., Mentinova, M., McLuckey, S.A.: Gas-phase conjugation to arginine residues in polypeptide ions via N-hydroxysuccinimide ester-based reagent ions. J. Am. Chem. Soc. 134, 11412–11414 (2012)
McGee, W.M., McLuckey, S.A.: Gas phase dissociation behavior of acyl-arginine peptides. Int. J. Mass Spectrom. 354, 181–187 (2013)
Hermanson, G.T.: Bioconjugation Techniques, 2nd edn. Academic Press, Amsterdam (2008)
Swaim, C.L., Smith, J.B., Smith, D.L.: Unexpected products from the reaction of the synthetic cross-linker 3,3′-dithiobis(sulfosuccinimidyl propionate), DTSSP with peptides. J. Am. Soc. Mass Spectrom. 15, 736–749 (2004)
Mädler, S., Bich, C., Touboul, D., Zenobi, R.: Chemical cross-linking with NHS esters: a systematic study on amino acid reactivities. J. Mass Spectrom. 44, 694–706 (2009)
Kalkhof, S., Sinz, A.: Chances and pitfalls of chemical cross-linking with amine-reactive N-hydroxysuccinimide esters. Anal. Bioanal. Chem. 392, 305–312 (2008)
Cuatrecasas, P., Parikh, I.: Adsorbents for affinity chromatography. Use of N-Hydroxysuccinimide esters of agarose. Biochemistry 11, 2291–2299 (1972)
Niles, R., Witkowska, H.E., Allen, S., Hall, S.C., Fisher, S.J., Hardt, M.: Acid-catalyzed oxygen-18 labeling of peptides. Anal. Chem. 81, 2804–2809 (2009)
Hager, J.W.: A new linear ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 16, 512–526 (2002)
Xia, Y., Liang, X., McLuckey, S.A.: Pulsed dual electrospray ionization for ion/ion reactions. J. Am. Soc. Mass Spectrom. 16, 1750–1756 (2005)
Xia, Y., Wu, J., McLuckey, S.A., Londry, F.A., Hager, J.W.: Mutual storage mode ion/ion reactions in a hybrid linear ion trap. J. Am. Soc. Mass Spectrom. 16, 71–81 (2005)
Londry, F.A., Hager, J.W.: Mass selective axial ion ejection from a linear quadrupole ion trap. J. Am. Soc. Mass Spectrom. 14, 1130–1147 (2003)
Gronert, S.: Gas phase studies of the competition between substitution and elimination reactions. Acc. Chem. Res. 36, 848–857 (2003)
Yoo, E.J.H., Feketeová, L., Khairallah, G.N., O’Hair, R.A.J.: Intercluster reactions shows that (CH3)2S+CH2CO2H is a better methyl cation donor than (CH3)3N+CH2CO2H. Eur. J. Mass Spectrom. 17, 159–166 (2011)
Stutzman, J.R., Blanksby, S.J., McLuckey, S.A.: Gas-phase transformation of phosphatidylcholine cations to structurally informative anions via ion/ion chemistry. Anal. Chem. 85, 3752–3757 (2013)
Prentice, B.M., Gilbert, J.D., Stutzman, J.R., Forrest, W.P., McLuckey, S.A.: Gas-phase reactivity of carboxylic acid functional groups with carbodiimides. J. Am. Soc. Mass Spectrom. 24, 30–37 (2013)
Acknowledgments
The authors thank Dr. Mingji Dai for helpful discussions on a potential gas-phase scrambling mechanism of the anhydride. This work was supported by the National Institutes of Health under grant GM 45372.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 174 kb)
Rights and permissions
About this article
Cite this article
Peng, Z., McGee, W.M., Bu, J. et al. Gas Phase Reactivity of Carboxylates with N-Hydroxysuccinimide Esters. J. Am. Soc. Mass Spectrom. 26, 174–180 (2015). https://doi.org/10.1007/s13361-014-1002-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-014-1002-0