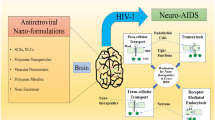
Abstract
Human immunodeficiency virus (HIV) is a neurotropic virus that enters the central nervous system (CNS) early in the course of infection. Although highly active antiretroviral therapy (HAART) has resulted in remarkable decline in the morbidity and mortality in AIDS patients, controlling HIV infections still remains a global health priority. HIV access to the CNS serves as the natural viral preserve because most antiretroviral (ARV) drugs possess inadequate or zero delivery across the brain barriers. The structure of the blood–brain barrier (BBB), the presence of efflux pumps, and the expression of metabolic enzymes pose hurdles for ARV drug-brain entry. Thus, development of target-specific, effective, safe, and controllable drug delivery approach is an important health priority for global elimination of AIDS progression. Nanoformulations can circumvent the BBB to improve CNS-directed drug delivery by affecting such pumps and enzymes. Alternatively, they can be optimized to affect their size, shape, and protein and lipid coatings to facilitate drug uptake, release, and ingress across the barrier. Improved drug delivery to the CNS would affect pharmacokinetic and drug biodistribution properties. This review focuses on how nanotechnology can serve to improve the delivery of antiretroviral medicines, termed NanoART, across the BBB and affect the biodistribution and clinical benefit for NeuroAIDS.
Similar content being viewed by others
Introduction
In 1981, Acquired Immune Deficiency Syndrome (AIDS) was first detected as a new disease when unusual opportunistic infections and rare malignancies are observed among young homosexual men [1], and its causative organism human immunodeficiency virus (HIV) was discovered in the year 1983 [2]. But, till today, AIDS is a chronic and often fatal disease of pandemic proportions. HIV infection results in cell-mediated immune deficiency through progressive loss and dysregulation of CD4+ T lymphocytes, leading to AIDS [3]. An effective and proper treatment for HIV-positive patients is still a dream to the doctors and scientists [4]. According to the US Centers for Disease Control and Prevention (CDC), AIDS may be defined by a CD4 count of <200 cells/μL or <14 % of all lymphocytes [5]. Once a person is infected with HIV, he should understand the progression of the disease from initial infection, then the latency period, symptomatic infections, and finally the AIDS. The life time of an untreated HIV-infected patient is not known but may go on for 10 years or more in many people. Several years into HIV infection, mild symptoms begin to develop, and then later severe infections that define AIDS occur.
Occurrence of retrovirus in numerous vertebrate species causes diversity of diseases. The retrovirus HIV which occurs in humans belongs to the subfamily Lentivirinae or slow viruses. The HIV is approximately 100 nm in diameter [6]. HIV contains two strands of RNA, enzyme, and an envelope with glycoprotein spikes. The envelope is termed as lipid membrane and the protein coat is termed as capsid (Fig. 1). Together, these molecules allow the virus to infect cells of the immune system and force them to build new copies of the virus [7]. The HIV viruses are classified into two types, type 1 and type 2 (HIV1, HIV2), which originated from the simian immunodeficiency viruses (SIVs) of primates. In 1983, HIV1 was first isolated and HIV2 in 1986, and they represent two different epidemics. HIV1 rose from the SIV of chimpanzees (SIVcpz) in humans, and HIV2 raised from the SIV of the sooty mangabey monkey (SIVsm) [6]. The HIV-1 strains can be classified into four groups: the “major” group M, the “outlier” group O, and two new groups, N and P, which are originated from four different cross-species transmissions from chimpanzees or gorillas to humans. The group M has caused the HIV pandemic whereas groups O, N, and P are mainly concentrated in Central Africa only [8, 9]. Among the total HIV-1 infections, around 90 % are caused by group M, and these are distributed worldwide. Group O infections are endemic to several west central African countries and covers 1 to 5 % of all HIV-1 infection in those countries. Group N has only been distinguished in a small number of population in Cameroon [10]. Within group M, there are nine genetically distinct subtypes (or clades) of HIV-1, viz., A, B, C, D, F, G, H, J, and K and inter-subtype circulating recombinant forms (CRFs); for example, the CRF A/B is a mixture of subtypes A and B [8, 9]. HIV-1 and HIV-2 strains can also be classified according to their phenotype. This phenotypic classification is related to cellular tropism for macrophages or T cell lines and to which kind of chemokine co-receptor the virus uses to gain entry into cells. Most NSI strains utilize the CCR5 co-receptor and are known as R5 viruses, whereas most SI strains utilize the CXCR4 co-receptor and are known as X4 viruses [11].
According to the WHO report, so far more than 39 million lives are affected by HIV and, in 2013, 1.5 million people died from HIV-related causes globally. It continues to be a prime global public health issue. At the end of 2013, there were approximately 35.0 million people who lived with HIV and 2.1 million people who become newly infected with HIV globally. Sub-Saharan Africa is the most affected region, with 24.7 million people living with HIV in 2013. Moreover, 70 % of the world’s newly affected HIV infections are coming from this region. It is well known that there is no complete cure for HIV infection. However, effective treatment with antiretroviral (ARV) agents can reduce the viral load so that HIV-infected people can enjoy healthy and productive lives. In 2013, among the HIV-infected people, globally 12.9 million people who survive with HIV had received antiretroviral therapy (ART), of which 11.7 million people were from low- and middle-income countries. The 11.7 million people taking ART represent 36 % of the 32.6 million people living with HIV in low- and middle-income countries. But still, the paediatric coverage is lagging in these countries. In 2013, one in three adults living with HIV received ART whereas less than one in four children living with HIV had access to ART [12]. In 2009, UNAIDS reported that more than 34 million people were infected with HIV, out of which more than 50 % show signs and symptoms of neuropsychiatric disorders. These disorders arise due to the effect of virus on the central nervous system (CNS) and peripheral nervous system (PNS) [13]. Approximately half of people with dementia belong to high-income countries, 39 % live in middle-income countries, and only 14 % live in low-income countries. The report predicts that, in 20 years, the numbers of people with dementia will double, expecting 65.7 million in 2030 and 115.4 million in 2050. This increment will be mostly from developing countries. The global standardized death rate in dementia for males is approximately 6.7 per 100,000 and 7.7 per 100,000 for females. According to the World Health Organization, mortality rate for dementia in India is 13.5 per 100,000 males and 11.1 for 100,000 females [14].
NeuroAIDS: mechanism, neuropathogenesis, and diagnosis
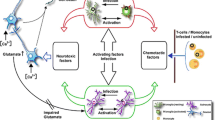
When HIV was initially isolated and characterized, it was believed to infect only CD4 lymphocyte, and the effects were restricted to the suppression of the immune system. However, in 1985, HIV was recovered from brain tissue, spinal cord, and peripheral nerves of the patient. This observation provided evidence of the role of HIV in causing primary infection of the brain [15]. HIV may enter into CNS either directly or as “Trojan passenger” via trafficking of infected monocytes, macrophages, and/or CD4+ T-cells across the tightly junctioned brain microvascular endothelial cells (BMVECs) of the blood–brain barrier (BBB). Early infection of HIV in the CNS triggers production of proteins that alter the BBB integrity (e.g., matrix metalloproteinase) and influence leukocyte transmigration across this barrier (e.g., monocyte chemotactic protein-1). These intensify the HIV infection resulting in the degradation of BBB leading to CNS injury. Many abusive drugs such as psychomotor stimulants (amphetamines), opiates (cocaine and morphine), alcohol, nicotine, and marijuana have been shown to promote susceptibility/progression of HIV infections and associated neuropathogenesis by altering the BBB, which leads to increased HIV entry. HIV directly infects and injures both systemic and innate central and peripheral nervous systems’ immune systems through infection respectively of T helper lymphocytes and of microglia, culminating in a wide spectrum of neuropsychiatric disorders or neuroAIDS. NeuroAIDS is associated with neurocognitive disorders such as HIV-associated dementia, minor neurocognitive disorder, mania/psychosis, anxiety, depression, seizures, mental slowness, forgetfulness, poor concentration, discombobulation, speech problem, decrease in spontaneity, myelopathy, and neuropathy with accompanying chronic neuropathic pain and physical disabilities [15–17]. In untreated AIDS, the brain was found to be vulnerable not only to opportunistic infections, which might or might not affect other organs, but also to a novel form of encephalitis induced by HIV itself [16]. These neuropsychiatric disorders are associated with diminished quality of life, increased health care costs, and reduced survival. Infection, in many cases, is a consequence of high-risk behaviors, including unprotected sex or injection drug use. Premorbid conditions such as addiction, mood disorders, anxiety disorders, and psychosis can place individuals at greater risk for high-risk behaviors and, consequently, for HIV infection. The medications used in the treatment of HIV also have their own associated side effects, of which some are neuropsychiatric. Efavirenz is a non-nucleoside reverse transcriptase inhibitor commonly used to treat HIV. It is associated with acute CNS side effects in 50 % of patients and has neuropsychiatric side effects when used chronically [17].
The underlying neurovirulent mechanisms for these disorders are thought to involve HIV infection of microglia and astrocytes (not neurons or oligodendrocytes) and have ensuing direct neurotoxic effects mediated by induced host-encoded molecules such as cytokines, proteases, reactive oxygen species, eicosanoids, excitotoxic amino acids (l-cysteine, glutamate, arachidonic acid), and neurotoxic actions exerted by virus-encoded proteins (e.g., gp120, Vpr, Tat) on proximate neurons [15–18]. Microglia are the only intrinsic brain cells to possess CD4 receptors, albeit in small numbers. These brain macrophages also bear chemokine receptors, principally CCR5, and are therefore potentially vulnerable to HIV infection once the virus has entered the brain compartment by crossing the BBB. Blood-derived monocytes migrate into the brain compartment under normal conditions, thereby contributing to the population of perivascular microglia which occupy the specialized microenvironment surrounding small blood vessels in the brain parenchyma. Activation of CD14+ and CD16+ monocytes outside the CNS promotes their penetration of the BBB. If these cells are also HIV infected, this transmigration into the CNS may constitute the earliest step in HIV neuroinvasion, thereby inducing the lymphocytic reaction observed in the brains of many presymptomatic HIV-infected individuals [16]. HIV-associated inflammatory cells and giant cells are often concentrated in the perivascular spaces around small blood vessels, particularly in the deep white matter, in the frontal cortex, and in the basal ganglia [15]. The cerebral cortical grey matter, the brain stem, and the cerebellum may all display evidence of HIV encephalopathy (HIVE). In other cases, the inflammation is widespread, conspicuous, and accompanied by considerable tissue damage, particularly in white matter which may be extensively demyelinated, with accompanying axonal damage, yet with only a few HIV-immunopositive microglia in occasional microglial nodules. Neuronal loss has been reported in AIDS, and this, together with synaptic and dendritic loss, likely contributes to the cognitive dysfunction of late stage disease although there is no exact correlation between the degree of dementia and the total amount of neuronal loss [16].
The year 1996 was a watershed year in the history of the HIV/AIDS epidemic [16]. More than 15 % of the world’s burden of disease is attributable to interlocking neurologic and psychiatric (neuropsychiatric) disorders. Neuropsychiatric disorders associated with HIV infection represent the convergence of a major global infectious epidemic with nervous system disease, resulting in substantial morbidity and mortality, especially as the disease progresses to AIDS [15–17].
HIV entry into the nervous system (neuroinvasion) occurs early after primary infection but persists throughout the disease course because the virus chronically infects glial cells (neurotropism) and has ensuing potential for nervous system disease (neurovirulence) [17]. HIV can be identified in brain tissue by a variety of methods, including immunohistochemistry, in situ hybridization, electron microscopy, and polymerase chain reaction (PCR) [16].
The clinical expression of this process includes neurocognitive impairments such as decreased attention/concentration, psychomotor speed, memory, learning, information processing, and executive function. There is also motor slowing, incoordination, and tremor, which may progress to disabling weakness, spasticity, extrapyramidal movement disorders, and paraparesis. In addition, there may be behavioral effects such as apathy and irritability. Psychomotor retardation (associated with damage to the frontal-striatal systems) also may occur. The clinical severity of this process ranges from asymptomatic neurocognitive impairment (ANI), to a mild neurocognitive disorder (MND), to full-blown HIV-associated dementia (HAD) [19].
Biomarkers are biological parameters that are objectively measured and quantifiable and that indicate changes in physiological states due to pathogenic processes or therapeutic intervention. Neuroproteomics reveals complex protein expression, function, interactions, and localization in cells of the nervous system. Six putative biomarkers—vitamin D binding protein, clusterin, gelsolin, complement C3, procollagen C-endopeptidase enhancer 1, and cystatin C—have been identified. Of these, vitamin D binding protein has been upregulated and the other five proteins have been downregulated in the cerebral spinal fluid (CSF) of HAD patients. SOD1 is an antioxidant and migration inhibitory factor secreted by macrophages during inflammation and an inhibitor of the protein kinases that are upregulated in the CSF of individuals with cognitive impairment. Proteomics has also been used to identify modifications of proteins in the CSF, providing clues to the pathogenesis of neuroAIDS; in particular, nitrosative/oxidative stress has been examined. Lipocalin-type prostaglandin D synthase (L-PDGS), a 3-nitrotyrosine (3-NT) protein, is a potential biomarker for neuroAIDS as enzymatic activity of this protein is significantly reduced with the HAD patient. Afamin, a member of the albumin superfamily, is significantly downregulated after HIV infection. Osteopontins (OPNs) increase in the CSF of HIV-infected individuals [20, 21].
Various neuroimaging like magnetic resonance imaging (MRI), computed tomography (CT), single-photon emission computed tomography (SPECT), and positron emission tomography (PET) studies indicate the presence of virus in the brain even during early stages of infection [15]. In vivo studies of HAND have utilized neuroimaging techniques including MRS, functional MRI (fMRI), and morphometry. MRS measures brain biochemistry, fMRI measures changes in blood flow related to neural activity in the brain, and morphometry models quantitative changes in neuroanatomical structures. These techniques also have been used to detect changes in the brains of asymptomatic HIV-1-positive patients [16, 19]. Magnetic resonance spectroscopy shows diminished N-acetyl-aspartate levels, a neuronal metabolite 37 together with increased choline levels indicative of inflammation [22].
NeuroAIDS research has relied heavily on neuropsychometric performance testing for both diagnosis and monitoring. Frequently used methods include versions of the mini-mental status examination, the montreal cognitive assessment, the composite Z scores of several brief neuropsychometric tests used in the AIDS Clinical Trials Group system, the International HIV dementia screen, and parts of Cog State devices [21].
HIV-associated dementia is not readily detected by the Folstein Mini-Mental Status Exam unless the patient is severely demented. More useful screening tools for HIV-associated dementia include the HIV Dementia Scale with a sensitivity and specificity exceeding 80 % and derivatives thereof, the Mental Alternation Test, the Executive Interview, and the HIV Dementia Assessment. The Memorial Sloan-Kettering Scale is a widely accepted tool for monitoring the progression and disability of dementia over time [22]. The schematic diagram of pathogenesis of neuroAIDS is shown in Fig. 2.
Implementation of ART has come up as respite for AIDS patients. Quite unrealistically, uninterrupted treatment for several years has been theorized for complete viral eradication with existing ART.
ART/HAART and neuroAIDS
Suppression of HIV replication is an important component to prevent HIV-associated morbidity and mortality as well as in improving the quality of life in patients with HIV infection. Adequate suppression requires strict adherence of ART [23]. Antiretroviral therapy is treatment for AIDS that helps the body’s immune system recover from the damage caused by infection with HIV. Although ART cannot cure AIDS, persons on ART will begin to feel better, eat more, and put on weight. Their bodies will recover the ability to fight infections. ARVs are powerful drugs. Once started, HIV ARV drugs must be taken for life, usually without any break in treatment, if they are to be effective [24]. Antiretroviral drugs are classified according to the step they inhibit in the viral life-cycle. US FDA has approved 25 anti-HIV drugs of different categories and fixed-dose combinations (FDCs) (Table 1). A milestone in the history of HIV disease has been the availability of new classes of drugs, in 1995–1996, allowing the introduction of combination ARV therapy (CART; also known as highly active antiretroviral therapy, or HAART) and the gradual evolution of HIV infection into a chronical, usually non-fatal condition [25]. The introduction of combination ART, compounded with the routine use of HIV RNA viral load and CD4+ T-cell counts as surrogate markers of drug efficacy and disease progression, has brought about a dramatic increase in life expectancy among HIV-infected patients [26]. CART typically comprises at least three antiretroviral drugs from two or more different classes. Multiple drugs are needed to inhibit HIV replication at several stages in the viral life cycle, reducing the likelihood of drug resistance [27]. Studies from several different centers have suggested that the brains of HAART-treated individuals display the premature appearance of insoluble proteins associated with neurodegeneration [16].
Failure of HAART in neuroAIDS
HAART therapy can decline plasma viral load below detection level resulting in 10-fold higher life expectancy. During the last decade, the mortality of AIDS patients have remarkably declined, and it is predicted that 50 % of HIV-infected people will cross the age of 50 years by 2015.
The morbidity is shown to decrease following the HAART treatment, but it is reported that at least 25 % of HAART-treated patients developed one or other neurological syndrome. This reduced efficacy of presently practiced HAART regimens for neuroAIDS can be attributed to many reasons. First, these treatments are not targeted for inflammatory cascades, thus HAART does not have a direct effect on the HIV-associated inflammatory degeneration. Second is the inadequate transmigration of ARV drugs across the brain barriers minimizing their detrimental efficacy on latent viral loads in the brain, which leads to generation of resistant viral strains against HAART. Third is the ultraselective permeability of BBB for xenobiotics. With the presence of efflux pumps and its high expression of metabolizing enzymes, the BBB is an effective barrier against many antiretrovirals. Thus, most ARV drugs remain impermeable across the brain barriers. In addition, ARVs are commonly proteins bound within the plasma, further limiting their access to the CNS. Fourth, ARV drugs have short half-life and low bioavailability, because of extensive first-pass metabolism including gastrointestinal degradation after oral administration. The expression of multidrug-resistant efflux proteins (MRPs) such as P-glycoprotein (P-gp) on the gastrointestinal tract further decreases their oral bioavailability. High doses are preferred because the treatment objective is to completely inhibit viral proliferation, an effect which is proportional to drug concentrations. The half-life for several ARV drugs is short, which then requires frequent administration of doses leading to poor patient compliance. The short residence time and reduced half-life of the drug in the plasma necessitate frequent administration of booster dosages as well as increased drug dosages contributing to the development of drug resistance. Fifth is long-term drug therapy. The nature of HIV infection, including viral persistence in reservoirs, necessitates long-term uninterrupted multi-drug ARV therapy. The lack of patient adherence to complicated drug administration regimens is further exacerbated by the cumulative costs of combined ARV therapy. Sixth is the drug toxicity and drug interactions. The long-term treatment with ARV drugs can create side-effects like constipation, fever, liver disorders, muscular dystrophy, metabolic disorders, and peripheral neuropathy. The use of a combination of drugs in the HAART therapy can lead to undesirable drug–drug interactions thereby reducing the efficacy of the drugs. In a combination therapy of nevirapine and saquinavir, the former drug induces the metabolism of the latter by cytochrome p450 leading to reduction of plasma level and efficacy of saquinavir. The emergence of various side effects and cost of HAART may also result in cessation of treatment. Collectively, the basic problem of HAART ineffectiveness in the treatment of neuroAIDS lies in the structural and functional complexity of brain barriers [28–30].
Brain/CNS is a very complex system; it provides a natural defense against toxic or infective agents circulating in the blood. The maintenance of the CNS homeostasis is essential for its normal function [31]. The human brain is a plastic organ constantly shaped by developmental processes and life’s experiences resulting in changes of the biochemical structure at the molecular and cellular level, thereby affecting information processing and flow [32]. Drug delivery to the brain is a challenge, because this tissue benefits from a very efficient protective barrier. The same mechanisms that protect the brain from foreign substances also restrict the entry of potentially active therapeutic moieties [33]. There are three barriers that limit drug transport to the brain parenchyma. These are the BBB, localized in the capillaries in the brain; the blood cerebrospinal fluid barrier (BCSFB), which is presented by the choroid plexus epithelium in the ventricles; and the ependymal, which is an epithelial layer of cells covering the brain tissue in the ventricles and limits the transport of compounds from the CSF to the brain tissue [34].
Blood–brain barrier
Ehrlich (1885) was the first to show evidence for the existence of a barrier between blood and brain [34]. BBB is a unique membranous barrier that tightly segregates the brain from the circulating blood. The CNS consists of blood capillaries, which are structurally different from the blood capillaries in other tissues. These structural differences result in a permeability barrier between the blood within brain capillaries and the extracellular fluid in brain tissue. Capillaries of the vertebrate brain and spinal cord lack the small pores that allow rapid movement of solutes from circulation into other organs. These capillaries are lined with a layer of special endothelial cells that lack fenestrations and are sealed with tight junctions (TJ) [35]. The TJs ultra-structurally appear as sites of apparent fusion involving the outer leaflets of plasma membrane of adjacent endothelial cells. The TJ consists of three integral membrane proteins, namely, claudin, occludin, and junction adhesion molecules, and a number of cytoplasmic accessory proteins including ZO-1, ZO-2, ZO-3, cingulin, and others cytoplasmic proteins link membrane proteins to actin, which is the primary cytoskeleton protein for the maintenance of structural and functional integrity of the endothelium [36]. Unlike peripheral capillaries that allow relatively free exchange of substances across/between cells, the BBB strictly limits transport into the brain through both physical (tight junctions) and metabolic (enzymes) barriers. Thus, the BBB is often the rate-limiting factor in determining permeation of therapeutic drugs into the brain [37]. Apart from this, a continuous uniform basement membrane surrounds the brain capillaries. This basal lamina encloses contractile cells called pericytes which form an intermittent layer and probably play some role in phagocytosis and defense, if the BBB is breached [38]. Astrocyte end-feet cover over 99 % of cerebral capillaries, leading to critical cell–cell interactions that directly modulate and regulate BBB characteristics. Several studies have demonstrated that astrocytes play a vital role in maintenance, and perhaps induction, of BBB characteristics, thereby producing an electrical resistance of 1500–2000 Ωcm2 much higher than that of the other systemic endothelia (3–33 Ωcm2) [39]. Although a well-established relationship exists between lipophilicity of a penetrant and the efficiency of brain penetration, there is a common misconception that small lipophilic molecules easily diffuse the BBB. In fact, some of these small solutes do not penetrate the brain as their lipid solubility may suggest. This phenomenon is due to the presence of some active transporters in BBB, more importantly the members of ATP-binding cassette (ABC) superfamily of transporters like P-gp, multidrug resistance protein (MRP), and breast cancer resistance protein (BCRP), which play crucial roles in active influx/efflux of the drugs regardless of the concentration gradient across the BBB [40]. Some transporters of nutrients such as glucose transporter (GLUT1) or large neutral amino acids (LAT1) have been used as transporter of some drugs and therefore enhance their uptake into the brain. The most widely used transporters for the delivery of large therapeutic compounds are transferrin receptor (TfR), LDL-related protein receptor (LPR), and insulin receptor [41].
Blood-CSF barrier
The blood-CSF (BCSF) barrier is formed by the choroid plexus (CP), the primary interface between the systemic circulation and the CSF. It is composed of fenestrated capillaries, which are joined together by TJs that link adjacent choroid plexus epithelial cells and limit paracellular diffusion of hydrophilic substances [39]. Considering its location and the direction of the CSF flow, the choroidal epithelium at the choroid plexus is considered the most important part of the BCSFB located in the lateral ventricles and in the third and fourth ventricles. The CP functions as a physical, enzymatic, and immunological barrier, and it plays a role in drug metabolism, drug transport, repair, and signalling [34]. However, the choroidal epithelial cells offer low resistance (150–200 Ωcm2) in comparison with capillary endothelial cells that form the BBB. As a result, various substances are able to move from the blood into the CSF in a molecular weight-dependent manner and irrespective of their movement across the BBB. For example, azidothymidine (AZT), an antiretroviral drug used for the treatment of HIV/AIDS, rapidly enters CSF across the choroid plexus epithelium but cannot easily cross the BBB [32]. Therefore, the BBB may be considered as the primary barrier that prevents the penetration of ARV drugs into the CNS. Thus, the active percentage of ARV drugs in the CNS remains at zero level or far below the pharmacological significant amount in most cases regardless of their permeability across the BBB.
Nano-ARTs and neuroAIDS
The complete lack of ARV therapies and ineffectiveness of HAART in the treatment of neuroAIDS has created a consistent global problem. Significant efforts have been made to design novel drug delivery systems (NDDS) for ARV drugs to reduce the dosing frequency, to enhance the bioavailability, to improve the CNS penetration, to inhibit the CNS efflux, and to deliver them to the target cells selectively with minimal side effects. The main aim of this drug delivery is to optimize a drug’s therapeutic index by strictly localizing its pharmacological activity to the site or organ of action resulting in a significant reduction in drug toxicity, reduction of the drug dose, and increased treatment efficacy.
Nanotechnology-based NDDS have shown great potential in the effective treatment of neuroAIDS. HIV nanotherapeutics has advantages of controlled and sustained release of the drug during transportation and at the site of localization, altering the organ distribution of the drug and subsequent clearance of the drug so as to achieve increase in drug therapeutic efficacy and reduction in side effects, toxicity, and adverse drug reactions. Site-specific targeting via receptor-mediated endocytosis can be achieved by attaching targeting ligands to the surface of the nanocarriers matching to a specific cell receptor or through the use of magnetic guidance. The nanocarriers loaded with specific P-gp efflux inhibitors can result in increased ARV drug concentration in the CNS. The system can be used for various routes of administration, including oral, nasal, and parenteral [42]. A significant amount of orally administered nanocapsulated drugs (<100 nm) escape the portal blood circulation route avoiding the reticuloendothelial digestion; rather, they are passed to systemic circulation via intestinal lymphatic transport resulting in remarkable reduction in the first-pass hepatic metabolism, which enhances their quantity and duration of bioavailability. They can freely flow into capillaries and remarkably increase in blood circulation time. The reduced first-pass hepatic metabolism and increased blood circulation time of nanoparticles make them suitable for the purpose of passive targeting. The accumulation of higher drug concentration at the BBB may enhance the drug permeability via passive diffusion. Nanocarriers for the treatment of neuroAIDS are small sized (1–100 nm) particles derived from various materials like polymer, lipid, metal, dendrimer, etc. They can be transported across the BBB without any damage to the BBB [43]. The enhanced transport mechanism may due to the following:
-
NPs open the tight junctions between endothelial cells and enable the drug to penetrate the BBB.
-
NPs are transcytosed through the endothelial cell layer.
-
NPs are endocytosed by endothelial cells and release the drug inside the cell.
-
Coating agents for NPs such as polysorbates inhibit the transmembrane efflux systems (i.e., P-gp) and increase BMVEC membrane fluidization and facilitated endocytosis [44]. The hydrophilic coating with polysorbates also improves the solubility of poorly soluble drugs.
The efficacy of nano-ART is further improved due to higher drug loading in nanocarriers with increased specific surface area. It results in initial burst release of drug leading to its active pharmacological level followed by a constant slow release for a prolonged time. Thus, the dose size and dosing frequency are minimized avoiding dose-related toxicities of ARV drugs. The present review focuses on how nanotechnology can serve to improve the delivery of antiretroviral medicines, termed nano-ART, across the BBB and affect the biodistribution and clinical benefit for neuroAIDS.
Polymeric nano-ARTs
The advancement of polymer chemistry and its application lead to the development of wide functional polymeric nanoparticles with precise archaeological control over its monomer, in order to transform into nanoparticles of selective overall sizes, shape, surface morphologies, and external surface charges. Synthetic polymers have the advantage of precise chemical composition and high predictable physical properties such as rate-controlled dissociation, degradation, erosion, permeability, and targeting efficacy [45]. The controlled and sustained drug delivery nanocarriers and devices are composed of polymer. Among the synthetic polymeric nanoparticles, some of them such as poly(lactic-co-glycolic acid) (PLGA) or polylactide (PLA), polymethacrylic acid, and polyethylene glycol (PEG) are biodegradable, where drugs are dissolved, entrapped, or encapsulated [46]. PLGA and PLA are degraded into glycolic acid and lactic acid, which are converted into water and carbon dioxide via tricarboxylic acid cycle and eventually eliminated from the body. Importantly, injection of these polyesters induces negligible and transient inflammatory response. Variety of drugs of both hydrophilic and hydrophobic nature can be entrapped on the matrix of PLA and PLGA. In addition, drug entrapment can be tailored for sustained release for a longer time. Surface modifications of these polyester polymers such as PEGlyation (attachment of poly (ethylene glycol)), agglutinin coating, and alginate embedding have been strongly recommended for delivery of a therapeutic dose across the BBB. Study on ARV drugs by Destache et al. [47] demonstrated that nanoformulations of ritonavir, lopinavir, and efavirenz with PLGA can maintain a sustaining peak of about 28 days in the mouse brain, which is limited to only days with free drugs [47]. Similarly, Rao et al. [48] demonstrated that at 2 weeks postadministration, PLA nanoparticles in conjugation with Tat peptides could result in 800-fold higher level of ritonavir in a mouse brain in comparison with drugs delivered in solution [48]. An in vitro study was done by Sumit and co-workers on colloidal gold-loaded, poly(d,l-lactic-co-glycolic acid)-based nanoparticles containing stavudine. To minimize the systemic toxicity of stavudine, providing reduced required drug dose and improved drug delivery over an extended period (63 days), macrophage targeted nanosystems were developed [49]. Nanoformulation of polybutylcyanoacrylate (PBCA) and methyl methacrylatesulfopropyl methacrylate (MMA-SPM) nanoparticles for brain targeting was investigated by Kuo and co-workers on several antiretroviral agents [50]. In a study, it was found that the permeation of zidovudine and lamivudine through the BBB was inversely proportional to the size of the nanoparticle prepared from PBCA and MMA-SPM [51]. Synthetic nanoparticle has been investigated for the delivery of zidovudine-lamivudine combination. The nanosystem was prepared through emulsion polymerization in a continuous aqueous phase of different polymers such as poly(lactic acid), PLGA, poly(methyl methacrylate) (PMMA), and methyl methacrylate sulfopropyl methacrylate (MMASPM). The author observed that as comparison to PLGA, other nanoparticles had a lower drug release. It is observed that the release of drug from PLGA nanoparticles was greater than 95 % within 10 h and acute toxicity to animal cells was not detected [47]. AZT was encapsulated on biodegradable poly(l-lactide) or poly(l-lactide)–poly(ethylene glycol) blend using the double-emulsion solvent-evaporation method. The authors reported that the presence of PEG influenced all of the analyzed physicochemical parameters and the drug release increases proportionately with the PEG presence in the blend [52]. Lamivudine was loaded in the nanoparticle of polymethyl acrylic acid. The drug was released from the nanoparticle in a slow and controlled manner with constant drug plasma concentration, resulting in increased therapeutic efficacy [53]. Like synthetic polymers, polymers from a natural origin such as cellulose, gelatine, pullulan, chitosan, alginate, and gliadin nanoparticles are typically used for biological applications. They are very biodegradable polymers where the biologic active agents can be dissolved, entrapped, or encapsulated. Unlike synthetic polymers, the natural polymers are diverse in physical as well as chemical composition [54–56]. Surfactant Tween 80-coated and uncoated chitosan nanoparticles containing lamivudine were prepared for targeted delivery to the brain. The system showed a very good stability for 60 days. Thus, it showed a cheaper and efficient carrier for targeting lamivudine to the brain for HIV-associated CNS disorders [57]. Jain and co-workers prepared mannosylated gelatin nanoparticles (MN-G-NPs), using a two-step desolvation technique. The cellular uptake by MN-G-NPs was 2.7 times more as compared with G-NPs. The results of this study demonstrated that coupling of the nanoparticles with mannose significantly enhanced the drug uptake by lung, liver, and lymph nodes, which is reflected by the presence of a higher percentage of drugs in these organs following administration of MN-G-NPs in comparison to non-coupled G-NPs or free drug [58].
Dendrimer nano-ARTs
Dendrimers are a versatile class of regularly branched macromolecules with unique structural and topologic features. They are characterized by very small size, typically less than 100 nm, narrow molecular weight distribution, and relative ease of incorporation of targeting ligands [54, 59]. These features make them potential and fascinating nanoparticles for the delivery of antiretroviral drugs. Unlike natural or synthetic polymers, they possess a highly branched three-dimensional architecture and are characterized by the presence of three different topologic sites such as the core, interior layers, and multifunctional surface [59, 60]. The polyfunctional core, surrounded by several layers of highly branched repeating units, such as polyethers, porphyrins, polyamidoamines, polyphenols, and polyamino acids, has the ability to encapsulate several chemical moieties. The multivalent surface interacts with the external environment with several functional groups. The physicochemical properties of dendrimers can be controlled during synthesis by selecting the core groups, the extent of branching, and the nature and/or number of functional groups on the surface. Dendrimers have been used as carriers of antiretroviral peptides and genes for HIV inhibition, and more surprisingly, many recent studies showed that they themselves can be used as antiretroviral agents [59, 61]. Dutta and co-workers loaded lamivudine into mannose-capped poly(propyleneimine) dendrimers. The authors reported a significant increase in antiretroviral activity, cellular uptake, and reduced cytotoxicity with respect to the free drug [62]. Researchers prepared efavirenz-loaded tuftsin conjugated 5th-generation poly(propyleneimine) dendrimers (TuPPI). Tuftsin is a natural macrophage activator tetrapeptide (Thr-Lys-Pro-Arg) able to bind specifically to mononuclear phagocytic cells enhancing their phagocytic activity. The authors reported that the dendrimer system is able to prolong the in vitro drug release up to 144 h with respect to 24 h of the PPI polymer. Moreover, a 34.5 times higher cellular uptake and reduced viral load by 99 % at a concentration of 0.625 ng/mL was reported; this activity was more significant in HIV-infected macrophages than uninfected cells [63]. A novel structure of silver complexes with anionic linear globular dendrimer was synthesized, characterized, and assessed against the HIV replication pathway in vitro. The result showed a very good yield of synthesis for the nanocomplex as well as a very potent antiretroviral activity with non-severe toxic effect [64]. Vacas-Córdoba and co-workers developed various combinations of anionic carbosilane dendrimers with sulfated (G3-S16) and naphthyl sulfonated (G2-NF16) ended groups with different ARVs against HIV-1 infection. The G3-S16 and G2-NF16 dendrimers showed a synergistic or additive activity profile with zidovudine, efavirenz, and tenofovir in the majority of the combinations tested against the X4 and R5 tropic HIV-1 in cell lines, as well as in human primary cells [65]. Jiménez et al. [66] investigated the potential of 2G-NN16 dendrimers (a carbosilane dendrimer) in in vitro BBB model for delivery of antiviral (HIV) small interfering RNA (siRNA). This siRNA/2G-NN16 dendriplexes showed permeability across the in vitro BBB and caused a significant reduction in the viral replication [66]. The limited application of dendrimers is associated with complicated production and their polycationic nature, which is toxic for negatively charged cell membranes resulting in cell death.
Vesicular nano-ARTs
Liposomes are vesicular carriers consisting of phospholipid bilayers surrounding an aqueous core. The aqueous core can be used to encapsulate hydrophilic drugs while hydrophobic and amphiphilic drugs can be solubilized within the phospholipid bilayers. Liposomes are usually small (80–100 nm), using a variety of encapsulation methods [67]. The unique and advantageous beneficial aspect of liposomes lies in their recognition as a foreign entity by the cells of the mononuclear phagocytic system (MPS), such as the monocytes/macrophages. Since HIV mainly resides in the macrophages and monocytes and can travel to the brain, the liposome-based nanoformulations of antiretrovirals can improve the efficacy of drugs and substantially reduce the side effects of the drugs both in vitro and in vivo [68]. Surface of liposomes can be easily modified to improve its properties [69], while incorporation of hydrophilic polyethylene glycol (PEG) can prevent interactions with plasma proteins, thus retarding recognition and removal by RES resulting in plasma circulation time. The antiviral effect and bone marrow toxicity of AZT-loaded liposomes in C57BL/6 mice were exploited. An improved antiviral activity in the infected mice was observed, compared to the free drug. Moreover, no bone marrow toxicity and enhanced localization of AZT in liver, spleen, and lung was reported [70]. Researchers investigated the macrophage uptake of stavudine in human monocyte/macrophage and explored the importance of charge on the liposomes in the cellular uptake. The uptake of liposomes containing phosphatidylserine was higher than that of liposomes prepared using dicetyl phosphate [71]. Kim et al. [72] investigated multi-vesicular liposomes for the delivery of the drug into the cerebrospinal fluid in a Sprague Dawley rat model. It was demonstrated that the half-life of lipo-zalcitabine in the brain of Sprague Dawley rats can be prolonged to 23 h as compared with 1.1 h for non-encapsulated drug [72]. Further, the superiority of the CNS-targeting ability of liposomes loaded with AZT-myristate (prodrug of AZT) was studied by Jin et al. [73]. It was shown that, with about 98 % encapsulation efficiency and longer half-life, a higher concentration of AZT was found in the brain and other organs of rats [73]. Researchers envisage the development of a stealth anti-CD4-conjugated immunoliposome containing two anti-retroviral drugs (nevirapine and saquinavir) that can selectively home into HIV-infected cells through the CD4 receptor. The drugs delivered via anti-CD4-conjugated immunoliposomes inhibited viral proliferation at a significantly lower concentration as compared to free drugs. Both drugs were found to localize in different regions of the liposome. The release of the reverse transcriptase inhibitor was dominant during the early phases of the release while in the later phases, the protease inhibitor is the major constituent released [74]. Despite the demonstrations that liposomal nano-ART can be highly effective in the treatment of neuroAIDS, several critical issues need to be addressed. The stability and leakiness of loaded drugs during storage and low drug entrapment ability of water-soluble drugs are the few areas still to be improved.
Biocompatible niosomes were fabricated using a biological surfactant, tyloxapol, with variable cholesterol concentrations encapsulating nevirapine. Formulation with a surfactant/cholesterol molar ratio of 1:0.1 exhibits maximum stability and optimum hydrophobicity. Such a versatile and improved formulation of NVP is expected to increase its therapeutic index and alleviate toxic systemic side effects while improving the quality of life and duration of survival of the patients [75].
Lipid nano-ARTs
Lipid NPs are nanometer-sized spheres surrounded by a lipid. Their lipophilic features facilitate crossing the BBB to enter the brain by endocytosis [76]. Several drugs have been incorporated into solid lipid NPs (SLNs), which are potentially useful for the treatment of brain diseases. SLNs have limited drug-loading capacities, but their advantages are as follows: (a) Unlike conventional bilayer liposomes, there is no random fusion between the particles or with other membranes. (b) Surface charge and molecular makeup can be easily modified with the possibility of multivalent attachment of small molecule ligands. They are made of one or more lipids with melting points higher than body temperature, so the carriers remain in solid state after administration. The low solubility of nanocarrier biomaterials probably contributes to the high tolerability of this formulation. A study showed that SLN in fact caused less non-specific cell toxicity even compared to nanoparticles made of PLGA, which has long been the standard for biocompatible materials [77]. Using a human brain microvessel endothelial cell line (hCMEC/D3) representative of the BBB, a significantly improved accumulation of [3H]-atazanavir was obtained when the drug was delivered by SLN. Cytotoxicity experiments indicate that SLN exhibits no toxicity in hCMEC/D3 cells up to a concentration corresponding to 200 nM of atazanavir [78]. Similarly, higher cellular accumulation of rhodamine-123, a substrate of efflux transporter P-gp, was also shown in this study. Thus, it was predicted that SLN may either mask or bypass the efflux pump [78]. Zidovudine palmitate-loaded SLNs prepared by Heiati et al. are the first reported antiretroviral SLNs. Trilaurin was used as the lipid core in these systems. Dipalmitoyl phosphatidylcholine alone or in combination with dimyristoylphosphatidylglycerol was used as a coating. The resultant SLNs were either neutral or negatively charged. Drug loading was dependent on the outer phospholipid coat, with higher phospholipid content resulting in greater drug incorporation [79]. Researchers prepared SLNs loaded with stavudine, delavirdine, and saquinavir independently and evaluated their ability to cross the BBB in vitro using human BMVECs. The entrapment efficiency of the drugs followed their lipophilicity, with the more lipophilic saquinavir having the maximum entrapment efficiency, indicating the better suitability of SLNs to more lipophilic drugs. The permeability of the drugs was improved 4–11-fold when incorporated into SLNs [80]. Surface charge modification is another approach that has been used to improve the targeted drug delivery of SLNs. Positively charged SLNs are found to deliver higher amounts of drugs to the brain than are uncharged or negatively charged SLNs. Kuo and Chen prepared cationic SLNs loaded with the lipophilic protease inhibitor, saquinavir. A blend of the nonionic lipids Compritol ATO 888 and cacao butter were used as core lipids. Stearylamine and dioctadecyldimethyl ammonium bromide comprised the peripheral cationic lipids. Polysorbate 80 was used as an SLN emulsifier/stabilizer [81]. Makwana and co-workers developed a lymph-targeted SLN formulation of efavirenz (EFV). SLN formulation was prepared using Gelucire 44/14, Compritol 888 ATO, Lipoid S 75, and Poloxamer 188 by hot homogenization technique followed by ultrasonication method, with a mean particle size of 168 nm, polydispersity index (PDI) <0.220, and mean zeta potential of −35.55 mV. A reduction in the amount (44.70 %) of the EFV reaching to the liver indicates that a major amount of EFV bypasses the liver and thereby enhances the oral bioavailability of the EFV. A significant amount of EFV was found in spleen, a major lymphatic organ. EFV SLN seems to have a potential to target the ARV to lymphatics for the better management of HIV [82]. Beloqui and co-workers prepared saquinavir-loaded NLC to enhance oral bioavailability. An NLC of size 247 nm and 1.5 % (w/v) surfactant content circumvented P-gp efflux and used both caveolae- and clathrin-mediated transcytosis. SQV transport across Caco-2 monolayers was enhanced up to 3.5-fold by NLCs compared to SQV suspension [83]. Vyas and co-workers explored facilitated transport of efavirenz across BBB using phenylalanine-anchored solid lipid nanoparticles (PA-SLN). SLNs (SLN and PA-SLN) were in nanometer size (around 150 nm) and possessed good entrapment efficiency (around 70 %). In vitro drug release revealed controlled release pattern for more than 24 h. In vivo studies showed 2–3-fold and 7–8-fold accumulation of PA-SLN in the brain as compared to SLN and EFV, respectively [84]. Alex and co-workers successfully encapsulated lopinavir in glyceryl behenate-based solid lipid nanoparticles (Lo-SLN) for its ultimate use to target intestinal lymphatic vessels in combined chemotherapy. SLNs with a mean particle size of 230 nm (PDI < 0.27) and surface electrical charge of approximate −27 mV were produced by hot homogenization process followed by ultrasonication. From the intestinal lymphatic transport study, it became evident that SLN increased the cumulative percentage dose of lopinavir secreted into the lymph compared with a conventional drug solution in methyl cellulose 0.5 % (w/v) as suspending agent [85]. The in vitro efficacy of various lipids nano-ART must be authenticated by in vivo studies before their application for human use.
Micelle nano-ARTs
A micelle is an aggregate formed by typically 50–100 amphiphilic molecules (e.g., surfactants, block-copolymers) when dispersed in a liquid phase [86]. In aqueous solution, the amphiphilic molecules aggregate and expose their hydrophilic heads outside and hide their hydrophobic segments in the inner core region. This structure facilitates solubilization of hydrophobic drug compounds within the micelle core. Three types of amphiphilic molecules, namely, block-copolymers, surfactants, and polymer-lipid conjugates, are used for formation of micelles [87]. Spitzenberger and co-workers demonstrated that administration of pluronic P85 alone or in combination with ART (zidovudine, lamivudine, and nelfinavir) resulted in 78–92 % reduction in the p24-expressing monocyte-derived macrophages (MDM) from the mouse brain compared to 62 % of ART only-treated group at 2 weeks postinoculation of HIV [88]. Sharma and Garg suggested that micelles may be tailored for highly selective active targeting by tethering hydrophilic block to ligands specific to HIV reservoir receptors such as lectin [89]. Peroni and co-workers encapsulated EFV within polymeric micelles made up of poloxamer Pluronic F127 and investigate whether the intestinal permeability of EFV is modulated by ABCG2 (breast cancer-resistant protein). They found that an ABCG2 inhibitor, fumitremorgin C (5–10 mM), significantly potentiated the mucosal-to-serosal permeation of the drug in everted gut sac; a 5-day oral treatment with 20 mg/kg EFV promotes the over-expression of ABCG2 in about 100 %, this phenomenon being accompanied by a clear decline in the intestinal permeability of the antiretroviral and the normalization of the ABCG2 expression within 24 h after the last administration of EFV was coincident with the recovery of the ability of the drug to permeate through the small intestine wall [90]. Chiappetta and co-workers developed a concentrated formulation of the first-line antiretroviral efavirenz by means of encapsulation within polymeric micelles. The aqueous solubility of the drug was increased more than 8400 times (up to 34 mg/mL). Despite the drug concentration and dose, micelles consistently resulted in significantly greater absorption rates, with PK parameters increasing up to 3-fold [91]. Jindal and co-workers developed a polymeric mixed micelle delivery system using Poloxamer 407 and Pluronic P123 for the encapsulation of an antiretroviral drug, nevirapine. The process of micellization of Poloxamer 407/Pluronic P 123 system has been found to be entropy dominant at low temperatures and enthalpy driven at high temperatures. The formulation at the 1:1 ratio exhibits high entrapment efficiency along with sustained release of the drug [92]. Sosnik and co-workers successfully synthesized thermo-sensitive graft copolymer amphiphiles of chitosan (CS) and poly(N-isopropylacrylamide)(PNiPAAm) (CS-g-PNIPAAm) by a catalyst-less one-pot gamma (γ)-radiation-assisted free radical polymerization. Polymeric micelle was prepared by this copolymer encapsulating indinavir. The critical micellar concentration(CMC) values were in the 0.0012–0.0025 % w/v range, and micelles displayed sizes between 99 and 203 nm with low polydispersity (<0.160) and highly positive zeta-potential(>+15 mV). Polymeric micelles led to a significant 24-fold increase of the aqueous solubility from 63 μg/mL to 1.45 mg/mL [93]. Chiappetta and co-workers investigated the intranasal administration of poly(ethelene oxide)–poly(propylene oxide) polymeric micelles loaded with efavirenz for targeting the CNS. The bioavailability of the drug in the CNS was increased 4-fold, and the relative exposure index (ratio between the area under the curve and plasma) was increased 5-fold with respect to the same administered intravenously [94]. The largest disadvantages of all types of micelles are their significant instability, which may reduce the circulation time resulting in premature drug release.
Magnetic nano-ARTs
Magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the most commonly used magnetic nanoparticles (MNPs) in the field of biomedicine. They have been extensively investigated for target-specific improved drug delivery. The main advantage that makes MNP superior over other counterparts such as liposomes, micelles, polymeric nanoparticles, etc., is that the unique superparamagnetic property can be utilized for simultaneous monitoring and quantitation of their tissue-specific or non-specific distribution. MNPs possess many characteristics essential for a suitable drug delivery nanocarrier. Synthesis of MNPs is quite easy, and it is feasible to produce monodispersed particles at the laboratory. The flexibility in the size of MNPs is described as follows: MNPs can respond to an external magnetic field. Thus, it is possible to “remote control” the movement of drug-loaded nanoparticles for target-specific delivery by applying the magnetic force at the exterior of the desired site [95]. In combination with the liposomes, MNPs can also be developed as hybrid nanoparticles called “magnetoliposomes.” Magnetoliposomes can be utilized for the monocytes/macrophage-based nanodrug delivery at the various inflammatory sites including the brain [96]. Magnetically guided drug targeting has been successfully demonstrated in various pathological cases including brain carcinomas, inflammations, etc. Also, MNPs as imaging agent have been used to diagnose brain-related anomalies. However, its application in the field of HIV/AIDS is limited. It was hypothesized that active 3′-azido-3′-deoxythymidine-5′-triphosphate (AZTTP) (a nucleotide analog reverse transcriptase inhibitors) may be directly immobilized on the surface of MNPs under the influence of ionic interaction leading the way for magnetic nanoformulations of ARV drugs. It was found that 1:0.2 ratios of MNPs to AZTTP give the best binding efficiency [97]. Researchers proposed MFH as a strategy to improve the killing of HIV-infected cells and for targeting the HIV-latent reservoirs like T-lymphocyte. Superparamagnetic iron oxide nanoparticle FeraSpin R was prepared and exhibited only limited toxicity, demonstrated an efficient uptake and cell-surface attachment, and only modestly impacted T-cell function [98]. Researchers developed magnetically guided layer-by-layer (LbL) assembled nanocarrier coencapsulation of an anti-HIV drug (tenofovir) and a latency-breaking agent (vorinostat) for the treatment of neuroAIDS. Nanoformulation showed good BBB transmigration ability with good in vitro antiviral efficacy over a period of 5 days after HIV infection in primary human astrocytes, with good cell viability [99]. It can be stated that magnetic nano-ART can reduce drug clearance, metabolism, and entrapment by reticuloendothelial system (RES).
Magneto electric nano-ARTs
Magnetic and electric fields depending on their intensity and frequency have been shown to exert therapeutic values for several diseases. Electric field-mediated therapies have been applied for the treatment of many CNS-related ailments such as pain, movement disorders, epilepsy, muscle stimulation, etc. However, little is known about the therapeutic values of coupling of magnetic and electric fields. Recently, researchers explored the potential of novel magneto-electric nanoparticles (MENPs) for targeted drug delivery and on-demand drug release of antiretroviral drugs across the in vitro BBB model. MENPs are a subgroup of multiferroic materials possessing a strong coupling ability of its magnetic and electric fields at body temperature. Similar to the MNPs, MENPs possess adequately high magnetic moments and, therefore, its movement could be “remote controlled” for its effective penetration across the BBB by applying external direct current (DC) magnetic field. Complementarily, unlike MNPs, its inherent non-zero electric property (AC trigger) could be used to controllably enforce the release of the bound drugs via breaking the symmetry of ionic bonding (charge distribution) between drug molecules and nanoparticles. Under the influence of remote low-energy DC, magnetic field ∼40 % of the MENP-bound AZTTP could translocate across the BBB, and this is ∼3 times higher than the free AZTTP. Applying magneto-electric filed did not alter the integrity of the in vitro BBB. More importantly, application of a very low AC field (44 Oe at 1000 Hz) resulted in nearly 100 % release of bound drugs from the particles. Owing to these extremely low-field magneto-electricity property MENPs enable dissipation (heat)-free mechanism and functional and structural integrity of the drugs and targeted cells remains unaffected. Thus, unlike other nanocarriers where drug release mechanism depends on “uncontrollable” cellular phenomena and pathology-specific responses, MENPs offer unique capability as a field-controlled drug carrier for on-demand release after crossing the BBB and could be relevant to the treatment of many other CNS and other diseases [29, 100].
Cell-based nano-ARTs
The inherent migratory potential of inflammatory-response cells (monocytes, macrophages, dendritic cells, neutrophils, lymphocytes, neuronal stem cells, bone-marrow derived mesenchymal stromal cells, etc.) towards the zone of inflammation can be exploited for the targeted drug delivery [29]. Although the use of immunocytes as drug carriers is still at a preliminary stage, it offers several advantages over use of traditional drug carriers. Immunocytes are spontaneously targeted to sites of injury, inflammation, and tumors; they can also serve as Trojan horses, carrying concealed payloads while migrating across impermeable barriers. In addition, this can diminish the immunogenicity and non-specific cytotoxicity of the drug cargoes. Drug-loaded nanomedicines, such as liposomes, magnetoliposomes, and nanoparticles, can be efficiently taken up by the immunocytes and then delivered to specific sites of injury for targeted therapy. Entry of drug-loaded nanovehicles in these cells is mediated by cell surface receptors such as mannose, complement, Fc receptors, etc. Thus, coating of nanocarriers with the receptor-specific moieties such as mannose, folate, gelatin, A-protein, RGD peptide, etc. complement the recognition by specific cell surface receptors leading to cellular internalization. Cell-mediated delivery of nanoformulated drugs is gaining significant consideration for the treatment of various brain diseases, specifically in chronic pathologies such as Alzheimer’s, Parkinson’s, brain cancer, epilepsy, etc. Its implications in HIV-related neuropathogenesis have also shown encouraging trends. Dou and co-workers demonstrated that macrophage-based nanoparticle platform can successfully deliver the active ARV drug in the brain. Indinavir formulated in suspensions of lipid nanocrystals were packaged into ex vivo cultivated bone marrow-derived macrophages and injected intravenously into severely combined immunodeficient HIV-1 encephalitis (HIVE) mice. High drug release in different regions of the brain was noticed consistently for at least 2 weeks, and a corresponding reduction in HIV replication was observed in the HIVE brain regions [101, 102]. To determine intracellular transfer of nano-ART-loaded MDM with a combination of atazanavir, ritonavir, indinavir, and efavirenz nano-ART, the nano-ART-loaded MDM were cultivated with endothelial cells. Nano-ART transfer from MDM to endothelial cells was observed in up to 52 % of cells, and folate coating of nano-ART further increased MDM to BMVEC particle transfer by up to 77 % [103]. Martinez-Skinner and co-workers developed long-acting nanoformulated antiretroviral therapy (nano-ART) which induces a range of innate immune migratory, phagocytic, and secretory cell functions that perpetuate drug depots. The localization of nano-ATV within leukocyte cell subsets was determined by confocal microscopy. ATV levels were highest at sites of injection in peritoneal or muscle macrophages, dependent on the injection site. The spleen and liver served as nano-ATV tissue depots while drug levels in lymph nodes were higher than those recorded in plasma [104].
Several critical questions regarding cell-based delivery of nano-ART need to be addressed. The first is whether MPs are capable of crossing the BBB once they are loaded with nano-ART. It has been demonstrated that MP-carrying nano-ART have no decrease in functional or migratory capabilities as measured by an in vitro model of the BBB. The second is whether these cells can reach disease-affected brain regions. It has been demonstrated that after adoptive transfer, the majority of macrophages accumulate in the lungs, liver, and spleen with a significant portion of cells which do in fact reach the brain and, once localized there, can persist and release the drug to the extracellular space for a long period of time. For example, BMM loaded with IDV-NPs adoptively transferred into an HIV-1 encephalitis mouse model can migrate across the BBB specifically to the diseased regions of the brain and release the drug for 14 days [101, 102]. The various antiretroviral compounds can be entrapped by a variety of carriers to make stable nano-ART that can be taken up by MPs and delivered to the brain. However, administration of these NPs is still an issue. To date, the only successful way to achieve macrophage delivery of nano-ART to diseased brain regions is by exposing the cells to the compounds ex vivo and then adoptively transferring them into another animal. This is not a viable method for clinical therapy. In order to make this method of drug delivery useful in humans, the nanoformulations of antiretroviral drugs must be able to specifically target monocytes and macrophages after systemic injection prior to removal from circulation.
Miscellaneous nano-ARTs
Cyclodextrins are a group of naturally occurring cyclic oligosaccharides composed of (1,4)-linked α-d-glucopyranose units. Topologically, cyclodextrins form a torus with a hydrophobic interior and a hydrophilic exterior. This allows cyclodextrins to act as host molecules that form inclusion complexes with hydrophobic guest molecules. Thus, cyclodextrin complexes can be used to enhance the solubility of lipophilic antiretroviral drugs, as well as to protect them from external degradation. Several classes of cyclodextrins, including β-cyclodextrin, methyl-β-cyclodextrin, and 2-hydroxypropyl-β-cyclodextrin, were studied for their ability to improve the solubility of the hydrophobic antiretroviral agents, efavirenz and UC781 [105, 106]. The faster in vitro dissolution rate profiles compared with the free efavirenz has been reported, which may result in higher rate of drug absorption after oral administration leading to higher drug uptake in the brain.
Nanoemulsions are kinetically stable transparent or translucent heterogeneous liquid dispersions which can exist as water-in-oil (w/o) or oil-in-water (o/w) forms. The internal phase liquid is reduced to droplets of ≤400 nm. Nanoemulsions are thermodynamically stable with very low surface tension, due to their small droplet size [107, 108]. The properties of the internal phase, including stability, are dependent on the composition of the surfactant present at the droplet interface. The choice of oil and surfactant will influence the in vitro as well as in vivo stability, transport, and release characteristics of the nanoemulsion formulation. Vyas et al. used an o/w nanoemulsion to improve the oral bioavailability and brain delivery of saquinavir [109]. The higher rate of absorption of the drug encapsulated in nanoemulsion compared to aqueous formulation resulted in higher brain uptake of saquinavir. Pereira et al. evaluated the brain delivery efficacy of indinavir-loaded lipid nanocapsule (IDV-LNC) compared to indinavir aqueous solution [110]. It was found that tissue/plasma ratios of LNC-loaded indinavir in the brain of normal (mdr1a+/+) or efflux transporter, P-gp-deficient (mdr1a−/−) mouse increased by 1.9 times on average as compared with indinavir in aqueous solution. At the same time, the ratio of aqueous indinavir in the brain of mdr1a−/− mouse was 21.3-fold higher than that of mdr1a+/+ mice suggesting that mechanisms other than, or additional to, P-gp inhibition may influence the higher uptake of LNC-loaded drugs [110].
Recently, carbon nanotubes have shown promise as anti-HIV-1 therapeutic. These carbon nanomaterials offer potential advantages over the more widely studied nanoparticle systems including their ability to cross cellular membranes and shuttle drugs, biomolecules including DNA, siRNA and proteins, into various types of cells such as cancer cells and T cells [111–114]. Their multimodal conjugation which allows the insertion of more than one type of functional group to the carbon nanotube surface may be a key property that establishes the superiority of nanopharmaceuticals over conventional agents. In addition, the presence of iron nanoparticles often encapsulated inside the carbon nanotubes allows their use as powerful magnetic carriers in drug delivery. Multiwalled carbon nanotubes conjugated with lamivudine and other antiretroviral agents have been investigated as anti-HIV agent [115]. Further in vitro and/or in vivo investigations can shed light on the legitimacy of their application for ARV drug delivery in CNS.
RNA-based therapies are an attractive prospect for the treatment of HIV. The siRNAs have anti-HIV-1 activity [116, 117] in cell cultures. The siRNA inhibits HIV infection by specifically degrading genomic HIV-1 RNA, thereby preventing formation of viral complementary DNA intermediates. siRNAs are hydrophilic polyanions with 21 base pairs that do not cross the cell membrane by diffusion and are not internalized upon docking to membrane receptors. RNA delivery to target sites, by vector and non-vector methods, has been plagued by several challenges that restrict the clinical utility of RNA therapeutics. These issues include rapid degradation in physiological conditions, short half-life, poor cellular uptake, subcellular compartmentalization, unwanted interactions with plasma proteins and the immune system, low bioavailability, and off-target side effects. Nucleic acid delivery by vector methods has also posed potential safety concerns due to their oncogenic, inflammatory, and immunogenic effects [118–127]. By preparing nanoparticulate electrostatic complexes between siRNA and polycations, the uptake and subsequent delivery of siRNA to intracellular compartments is ensured [128–131]. The cationic reagent (which may be a peptide, liposome, or dendrimer) protects the nucleic acid against degradation and facilitates cellular uptake by endocytosis [132]. Amino-terminated carbosilane dendrimers formed dendriplexes with siRNA, which were delivered to human astrocytes, where they reduced the replication of HIV-1 [133]. The siRNA administered to humanized mice using immunoliposome nanoparticles resulted in selective uptake of siRNA by T-cells and macrophages and reduction in HIV plasma viral load [134]. Reynolds et al. [135] reported application of gold nanoparticle-mediated delivery of siRNA against galectin-1 (an adhesion molecule) in methamphetamine-treated, HIV-infected MDM. They showed that the stimulatory effect of methamphetamine on gelatin-1 gene expression is countered because of siRNA knockdown, and concomitantly, HIV infection is attenuated [135]. Some of the important nano-ART developed is listed in the table mentioned below (Table 2).
Patented nano-ARTs (Table 3)
Deastache filed a patent on nanoformulation of ART using three antiretroviral agents in combination. He found the intracellular concentration of drugs up to a level that is at least IC50 against HIV. This concentration within the cell is maintained up to 28 days after administration [143]. Chaubal et al. filed a patent on nanosuspensions of indinavir for increased CNS delivery. He prepared nanosuspensions of anti-retroviral agents by the process of microprecipitation and energy addition method. The nanosupensions of the present invention can deliver anti-retroviral agent to the brain to treat HIV infection [144]. Boger et al. filed a patent on nanosized aspartyl protease inhibitors. They solve the problem of low solubility of aspartyl protease inhibitors by decreasing the size to nanolevel. Applicants wet-milled the free base form of the aspartyl protease inhibitor in the presence of a surface modifier (nanosized particles). They found that the resulting composition was stable, dispersible with a bioavailability equal to or greater than a “solution” form of the corresponding salt form of the same inhibitor [145]. Destache et al. filed a patent on polymeric nanoparticle in a thermosensitive gel for coital independent vaginal prophylaxis of HIV. They prepared efaverinz- and raltegravir-loaded PLGA nanoparticle and found that efaverinz maintains the drug concentration up to 10 days and raltegravir upto 6 days [146].
Conclusion and future scopes
Since the earliest studies in neuroAIDS, nearly 30 years ago, many different approaches and models for neuroAIDS have been evolved. CNS infection by HIV-1 can result in neuroAIDS. It is demonstrated that HAART greatly reduces peripheral viral load by decreasing viral replication but has little effect on the viral reservoirs in the brain. After more than 20 years of research exploring the mechanisms behind HIV spread to the brain, viral perpetuation and neuronal degeneration have provided many targets for therapy. However, this condition remains particularly difficult to treat since the BBB is very effective at keeping antiretroviral drugs out of the brain. Getting therapeutic compounds across the BBB and into the brain has also been a major goal for the treatment of neuroAIDS. With the advent of nanotechnology, new tools have been made available for accomplishing this goal. Nanocarriers with unique physical and chemical properties allow for loading, entrapment, protection, and transport of ARV drugs. As technology advances, it is now becoming possible to target nano-ART to specific cells, such as BMVECs or MPs, or to avoid the reticuloendothelial system altogether. This allows either vastly extending the circulation time of these drugs or pinpointing them to specific regions. A variety of nanocarriers such as dendrimers, polymer-NPs, SLNs, liposomes, nanocrystals, nanoemulsions, and nanocapsules are being used to transport antiretroviral drugs to the brain. However, none of the research described here has proceeded beyond the preclinical stage. The success of any nanopharmaceutical depends on at least three criteria, none of which can be satisfactorily or completely assessed without clinical data: Firstly, the nanopharmaceutical should exert an antiviral effect; secondly, it should have an acceptable toxicity profile; and thirdly, it should be stable and be able to overcome biological barriers. The challenge is that optimizing one criterion may be detrimental to the others; e.g., optimizing efficacy (by, for instance, including an additional therapeutic agent into a multifunctional nanoparticle) may exacerbating toxicity (because such an agent may be toxic) and decreasing stability (because the more complex construct is likely to be less stable) [147]. The relevant research homework has to be elucidated more rigorously to sort out the various associated shortcomings of this novel approach in the treatment of neuroAIDS. Assays to assess safety and efficacy need to be standardized and validated. Computerized models, which predict or simulate nano-ART behavior, need to be developed and optimized [148]. The selection of safe (non-toxic and biodegradable) nanocarriers, development of specific targeting strategies, multifunctionalization of nanocarriers, and more realistic in vivo experimentations are few areas that should be given importance to enhance the feasibility of nano-ARTs to treat the AIDS-related neuropathogenesis. The very specific targeted nanocarriers may lead to suboptimal level of drug in the non-targeted tissues selecting out of the drug-resistant viral strain there. Also, the targeted nano-ARTs with single antiretroviral drug will effectively select out the resistant virus in the targeted tissues. The development of multifunctional nano-ARTs may be the key issue that establishes the superiority of nanomedicines in neuroAIDS. Eventually, proper pharmacokinetic and pharmacodynamic studies and large-scale manufacturization will lead to successful application of nano-ARTs in neuroAIDS patients in more realistic clinical settings, eliminating viral reservoirs in the brain.
References
Paul MS, Beatrice HH. Origins of HIV and the AIDS pandemic. Cold Spring Harbor Perspect Med. 2011;1:1–22.
FDA-Approved HIV Medicines. 2015. https://aidsinfo.nih.gov/education-materials/fact-sheets/21/58/fda-approved-hiv-medicines. Accessed 25 Dec 2015.
Friedrich BM, Dziuba N, Li G, Endsley MA, Murray JL, Ferguson MR. Host factors mediating HIV-1 replication. Virus Res. 2011;161:101–14.
Biswas MHA. AIDS epidemic worldwide and the millennium development strategies: a light for lives. HIV AIDS Rev. 2012;11:87–94.
Hare CB. Clinical Overview of HIV Disease. 2009. http://hivinsite.ucsf.edu/InSite?page=kb-03-01-01#S7.2X. Accessed 25 Dec 2015.
Weiss RA, Dalgleish AG, Loveday C, Pillay D. Human immunodeficiency viruses. In: Zuckerman AJ, Banatvala JE, Pattison JR, Griffiths PD, Schoub BD, editors. Principles and practice of clinical virology. 5th ed. New York: John Wiley & Sons Ltd; 2004. p. 721–57.
Seiter J, Fass M, Stanley E, Waterman M. HIV/AIDS: Biology and Treatment; Global Health has No Borders: Case Investigations in Biology and Global Health. Biology International. 2011; 49:86–95. http://biologyinternational.org/wp-content/uploads/2011/09/Vol49-2rev.pdf.
HIV Strains: Types, Groups and Subtypes. http://www.avert.org/hiv-types.htm. Accessed 25 Dec 2015.
Abecasis AB, Wensing AMJ, Paraskevis D, Vercauteren J, Theys K, Vijver DAMCVD, et al. HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology. 2013;10:1–13.
HIV-1 subtypes. http://www.aidsmap.com/HIV-1-subtypes/page/1322996/. Accessed 25 Dec 2015.
Gabuzda D, Wang J. Chemokine receptors and virus entry in the central nervous system. J Neuro Virol. 1999;5:643–58.
HIV/AIDS. 2015. http://www.who.int/mediacentre/factsheets/fs360/en/. Accessed 25 Dec 2015.
Verma AS, Singh UP, Dwivedi PD, Singh A. Contribution of CNS cells in neuroAIDS. J Pharm Bioallied Sci. 2010;2:300–6.
Saxena SK, Tiwari S, Nair MPN. NeuroAIDS: Mechanisms, Causes, Prevalence, Diagnostics and Social Issues. In: Saxena SK, editor. Current Perspectives in HIV Infection. InTech; 2013. Pp. 109–124. doi:10.5772/55100.
Sharma D, Bhattacharya J. Cellular & molecular basis of HIV-associated neuropathogenesis. Indian J Med Res. 2009;129:637–51.
Bell JE, Anthony IC, Simmonds P. The changing pathology of NeuroAIDS associated with drug abuse in the era of HAART. Am J Infect Dis. 2006;2:39–48.
McCombe JA, Noorbakhsh F, Buchholz C, Trew M, Power C. NeuroAIDS: a watershed for mental health and nervous system disorders. J Psychiatry Neurosci. 2009;34:83–5.
Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales, Koob GF, et al. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 2014;9:1–15.
Shapshak P, Kangueane P, Fujimura RK, Commins D, Chiappelli F, Singer E, et al. Editorial: NeuroAIDS review. AIDS. 2011;25:123–41.
Pendyala G, Fox HS. Proteomic and metabolomic strategies to investigate HIV-associated neurocognitive disorders. Genome Med. 2010;2:1–7.
Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–86.
Power C, Boissé L, Rourke S, Gill MJ. NeuroAIDS: an evolving epidemic. Can J Neurol Sci. 2009;36:285–95.
Kumarasamy N, Patel A, Pujari S. Antiretroviral therapy in Indian setting: when & what to start with, when & what to switch to? Indian J Med Res. 2011;134:787–800.
Hope R, Israel E. The Essentials of Antiretroviral Therapy for Health Care and Program Managers. Technical guidance. Series No. 5. Pathfinder International; 2007.
Palmisano L, Vella S. A brief history of antiretroviral therapy of HIV infection: success and challenges. Ann Ist Super Sanita. 2011;47:44–8.
Esté JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antivir Res. 2010;85:25–33.
Ellis R. HIV and antiretroviral therapy: impact on the central nervous system. Prog Neurobiol. 2010;91:185–7.
Ruiz A, Nair M, Kaushik A. Recent update in NanoCure of NeuroAIDS. Sciencejet. 2015;4:1–9.
Sagar V, Kanthikeel SP, Pottathil R, Saxena SK, Nair M. Towards nanomedicines for neuroAIDS. Rev Med Virol. 2014;24:103–24.
Ganau M, Prisco L, Pescador D, Ganau L. Challenging new targets for CNS–HIV infection. Front Neurol. 2012;3:1–4.
Peluffo H, Unzueta U, Demontel MLN, Xu Z, Váquez E, Miralles NF, et al. BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS. Biotechnol Adv. 2015;33:277–87.
Grabrucker AM, Chhabra R, Belletti D, Forni F, Vandelli MA, Ruozi B, et al. Nanoparticles as Blood–Brain Barrier permeable CNS targeted drug delivery systems. Topics Med Chem. 2004;10:71–89.
Karanth H, Murthy RSR. Nanotechnology in brain targeting. Int J Pharm Sci Nanotechnol. 2008;1:9–24.
Boer AGD, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol. 2007;47:323–55.
Misra M, Ganesh S, Shahiwala A. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6:252–73.
Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13.
Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127:97–109.
Pathan SA, Iqbal Z, Zaidi SMA, Talegaonkar S, Vohra D, Jain GK, et al. CNS drug delivery systems: novel approaches. Recent Patents Drug Deliv Formulation. 2009;3:71–89.
Covarrubias LS, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr Pharm Des. 2014;20:1422–49.
Mehdipour AR, Hamidi M. Brain drug targeting: a computational approach for overcoming blood–brain barrier. Drug Discov Today. 2009;14:1030–6.
Abbott N, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37:13–25.
Iannazzo D, Pistone A, Romeo R, Giofrè SV. Nanotechnology approaches for antiretroviral drugs delivery. J AIDS HIV Infect. 2015;1:1–13.
Jain KK. Nanobiotechnology-based drug delivery to the central nervous system. Neurodegener Dis. 2007;4(4):287–91.
Barbu E, Molnàr E, Tsibouklis J, Górecki DC. The potential for nanoparticle-based drug delivery to the brain: overcoming the blood–brain barrier. Expert Opin Drug Deliv. 2009;6(6):553–65.
Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–61.
Zhang J, Li S, Li X. Polymeric nano-assemblies as emerging delivery carriers for therapeutic applications: a review of recent patents. Recent Patents Nanotechnol. 2009;3:225–31.
Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA. Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice. J Antimicrob Chemother. 2010;65(10):2183–7.
Rao KS, Reddy MK, Horning JL, et al. TATconjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–38.
Basu S, Mukherjee B, Chowdhury SR, Paul P, Choudhury R, Kumar A, et al. Colloidal gold-loaded, biodegradable, polymer-based stavudine nanoparticle uptake by macrophages: an in vitro study. Int J Nanomedicine. 2012;7:6049–61.
Kuo YC. Loading efficiency of stavudine on polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate copolymer nanoparticles. Int J Pharm. 2005;290:161–72.
Kuo YC, Chen HH. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood–brain barrier. Int J Pharm. 2006;327:160–9.
Mainardes RM, Gremiao MP. Nanoencapsulation and characterization of zidovudine on poly(L-lactide) and poly(L-lactide)-poly(ethylene glycol)-blend nanoparticles. J Nanosci Nanotechnol. 2012;12:8513–21.
Tamizhrasi S, Shukla A, Shivkumar T, Rathi V, Rathi JC. Formulation and evaluation of lamivudine loaded polymethacrylic acid nanoparticles. Int J PharmTech Res. 2009;1:411–5.
Mallipeddi R, Rohan LC. Progress in antiretroviral drug delivery using nanotechnology. Int J Nanomedicine. 2010;5:533–47.
Mahajan SD, Aalinkeel R, Law W-C, Reynolds JL, Nair BB, Sykes DE, et al. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int J Nanomed. 2012;7:5301–14.
Ramana LN, Sharma S, Sethuraman S, Ranga U, Krishnan UM. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim Biophys Acta. 1840;2014:476–84.
Dhanya KP, Santhi K, Dhanaraj SA, Sajeeth CI. Formulation and evaluation of chitosan nanospheres as a carier for the targeted delivery of lamivudine to the brain. Int J Comprehensive Pharm. 2011;2:1–5.
Jain SK, Gupta Y, Jain A, Saxena AR, Khare P, Jain A. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine. 2008;4:41–8.
Peng J, Wu Z, Qi X, Chen Y, Li X. Dendrimers as potential therapeutic tools in HIV inhibition. Molecules. 2013;18:7912–29.
Svenson S, Tomalia DA. Dendrimers in biomedical applications-reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–29.
Macri RV, Karlovska J, Doncel GF, Du X, Maisuria BB, Williams AA, et al. Comparing anti-HIV, antibacterial, antifungal, micellar, and cytotoxic properties of tricarboxylato dendritic amphiphiles. Bioorg Med Chem. 2009;17:3162–8.
Dutta T, Jain NK. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biophys Acta. 2007;1770:681–6.
Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly (propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur J Pharm Sci. 2008;34:181–9.
Ardestani MS, Fordoei AS, Abdoli A, Ahangari Cohan R, Bahrali G, Sadat SM, et al. Nanosilver based anionic linear globular dendrimer with a special significant antiretroviral activity. J Mater Sci Mater Med. 2015;26(5):179.
Vacas-Córdoba E, Galán M, de la Mata JF, Gómez R, Pion M, Muñoz-Fernández MA. Enhanced activity of carbosilane dendrimers against HIV when combined with reverse transcriptase inhibitor drugs: searching for more potent microbicides. Int J Nanomedicine. 2014;9:3591–600.
Jiménez JL, Clemente MI, Weber ND, et al. Carbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virus. BioDrugs. 2010;24:331–43.
Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv Drug Deliv Rev. 2010;62(4–5):503–17.
Lanao JM, Briones E, Colino CI. Recent advances in delivery systems for anti-HIV1 therapy. J Drug Target. 2007;15:21–36.
Gunaseelan S, Gunaseelan K, Deshmukh M, Zhang X, Sinko PJ. Surface modifications of nanocarriers for effective intracellular delivery of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62(4–5):518–31.
Phillips NC, Skamene E, Tsoukas C. Liposomal encapsulation of 3′-azido-3′- deoxythymidine (AZT) results in decreased bone marrow toxicity and enhanced activity against murine AIDS-induced immunosuppression. J Acquir Immune Defic Syndr. 1991;4:959–66.
Katragadda A, Bridgman R, Betageri G. Effect of liposome composition and cholesterol on the cellular uptake of stavudine by human monocyte/ macrophages. Cell Mol Biol Lett. 2000;5:483–93.
Kim S, Scheerer S, Geyer MA, Howell SB. Direct cerebrospinal fluid delivery of an antiretroviral agent using multivesicular liposomes. J Infect Dis. 1990;162:750–2.
Jin SX, Bi DZ, Wang J, Wang YZ, Hu HG, Deng YH. Pharmacokinetics and tissue distribution of zidovudine in rats following intravenous administration of zidovudine myristate loaded liposomes. Pharmazie. 2005;60:840–3.
Ramana LN, Sharma S, Sethuraman S, Ranga U, Krishnan UM. Stealth anti-CD4 conjugated immunoliposomes with dual antiretroviral drugs—modern Trojan horses to combat HIV. Eur J Pharm Biopharm. 2015;89:300–11.
Mehta SK, Jindal N. Tyloxapol niosomes as prospective drug delivery module for antiretroviral drug nevirapine. AAPS PharmSciTech. 2015;16(1):67–75.
Bondì ML, Di Gesù R, Craparo EF. Lipid nanoparticles for drug targeting to the brain. Methods Enzymol. 2012;508:229–51.
Muller RH, Ruhl D, Runge S, Schulze-Forster K, Mehnert. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surface. Pharm Res. 1997;14:458–62.
Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25:2262–71.
Heiati H, Tawashi R, Shivers RR, Phillips NC. Solid lipid nanoparticles as drug carriers. I. Incorporation and retention of the lipophilic prodrug 3′-azido-3′-deoxythymidine palmitate. Int J Pharm. 1997;146(1):123–31.
Kuo Y-C, Su F-L. Transport of stavudine, delavirdine, and saquinavir across the blood–brain barrier by polybutylcyanoacrylate, methylmethacrylate- sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm. 2007;340(1–2):143–52.
Kuo Y-C, Chen H-H. Entrapment and release of saquinavir using novel cationic solid lipid nanoparticles. Int J Pharm. 2009;365(1–2):206–13.
Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495:439–46.
Beloqui A, Solinís MA, Gascón AR, Pozo-Rodríguez AD, Rieux AD, Préat PV. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J Control Release. 2013;166:115–23.
Vyas A, Jain A, Hurkat P, Jain A, Jain SK. Targeting of AIDS related encephalopathy using phenylalanineanchored lipidic nanocarrier. Colloids Surf B: Biointerfaces. 2015;131:155–61.
Alex MRA, Chacko AJ, Jose S, Souto EB. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci. 2011;42:11–8.
Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19:1–72.
Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine. 2010;5:485–505.
Spitzenberger TJ, Heilman D, Diekmann C, Batrakova EV, Kabanov AV, Gendelman HE, et al. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. J Cereb Blood Flow Metab. 2007;27:1033–42.
Sharma P, Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62:491–502.
Peroni RN, Di Gennaro SS, Hocht C, Chiappetta DA, Rubio MC, Sosnik A, et al. Efavirenz is a substrate and in turn modulates the expression of the efflux transporter ABCG2/BCRP in the gastrointestinal tract of the rat. Biochem Pharmacol. 2011;82:1227–33.
Chiappetta DA, Hocht C, Taira C, Sosnik A. Oral pharmacokinetics of the anti-HIV efavirenz encapsulated within polymeric micelles. Biomaterials. 2011;32:2379–87.
Jindal N, Mehta SK. Nevirapine loaded Poloxamer 407/Pluronic P123 mixed micelles: optimization of formulation and in vitro evaluation. Colloids Surf B: Biointerfaces. 2015;129:100–6.
Sosnik A, Imperialeb JC, Vázquez-González B, Raskin MM, Mu˜noz-Mu˜noz F, Burillo G, et al. Mucoadhesive thermo-responsivechitosan-g-poly(N-isopropylacrylamide) polymeric micelles via aone-pot gamma-radiation-assisted pathway. Colloids Surf B: Biointerfaces. 2015;136:900–7.
Chiappetta DA, Hocht C, Opezzo JA, Sosnik A. Intranasal administration of antiretroviral-loaded micelles for anatomical targeting to the brain in HIV. Nanomedicine. 2013;8(2):223–37.
Pan Y, Du X, Zhao F, Xu B. Magnetic nanoparticles for the manipulation of proteins and cells. Chem Soc Rev. 2012;41:2912–42.
Saiyed ZM, Gandhi NH, Nair MPN. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood–brain barrier. Int J Nanomedicine. 2010;5(1):157–66.
Sun J, Li Y, Liang X-J, Wang PC. Bacterial magnetosome: a novel biogenetic magnetic targeted drug carrier with potential multifunctions. J Nanomater. 2011;2011(2011):469031–43.
Williams JP, Southern P, Lissina A, Christian HC, Sewell AK, Phillips R, et al. Application of magnetic field hyperthermia and superparamagnetic iron oxide nanoparticles to HIV-1-specific T-cell cytotoxicity. Int J Nanomedicine. 2013;8:2543–54.
Jayant RD, Atluri VSR, Agudelo M, Sagar V, Kaushik A, Nair M. Sustained-release Nano-ART formulation for the treatment of Neuro-AIDS. Int J Nanomedicine. 2015;10:1077–93.
Nair M, Guduru R, Liang P, Hong J, Sagar V, Khizroev S. Externally-controlled on-demand release of anti-HIV drug AZTTP using magneto-electric nanoparticles as carriers. Nat Commun. 2013;4:1707.
Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8:415–33.
Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of Neuro-AIDS. J Immunol. 2009;183(1):661–9.
Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, et al. Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int J Nanomedicine. 2012;7:2373–88.
Martinez-Skinner AL, Araínga MA, Puligujja P, Palandri DL, Baldridge HM, Edagwa BJ, McMillan JM, Mosley RL, Gendelman HE; Cellular Responses and Tissue Depots for Nanoformulated Antiretroviral Therapy. PLOS ONE, 2015; 1–19.
Sathigari S, Chadha G, Lee YH, et al. Physicochemical characterization of efavirenz-cyclodextrin inclusion complexes. AAPS PharmSciTech. 2009;10(1):81–7.
Yang H, Parniak MA, Isaacs CE, Hillier SL, Rohan LC. Characterization of cyclodextrin inclusion complexes of the anti- HIV non-nucleoside reverse transcriptase inhibitor UC781. AAPS J. 2008;10(4):606–13.
Vyas TK, Shah L, Amiji MM. Nanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sites. Expert Opin Drug Deliv. 2006;3(5):613–28.
Sarker DK. Engineering of nanoemulsions for drug delivery. Curr Drug Deliv. 2005;2(4):297–310.
Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347(1–2):93–101.
Pereira M, de Oliveira E, Garcion NV, Benoit JP, Couet W, Olivier JC. Tissue distribution of indinavir administered as solid lipid nanocapsule formulation in mdr1a(+/+) and mdr1a (−/−) CF-1 mice. Pharm Res. 2005;22:1898–905.
Iannazzo D, Piperno A, Pistone A, Grassi G, Galvagno S. Recent advances in carbon nanotubes as delivery systems for anticancer drugs. Curr Med Chem. 2013;20:1333–54.
Cunha C, Panseri S, Iannazzo D, Piperno A, Pistone A, et al. Hybrid composites made of multiwalled carbon nanotubes functionalized with Fe3O4 nanoparticles for tissue engineering applications. Nanotechnology. 2012;23:465102.
Iannazzo D, Piperno A, Ferlazzo A, Pistone A, Milone C, et al. Functionalization of multi-walled carbon nanotubes with coumarin derivatives and their biological evaluation. Org Biomol Chem. 2012;10:1025–31.
Liu Z, Winters M, Holodniy M, Dai H. SiRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew Chem Int Ed. 2007;46:2023–7.
Iannazzo D, Pistone A, Galvagno S, Ferro S, De Luca L, et al. Synthesis and anti-HIV activity of carboxylated and drug-conjugated multi-walled carbon nanotubes. Carbon. 2015;82:548–61.
Jacque JM, Triques K, Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature. 2002;418:435–8.
Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK, et al. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8:681–6.
Fattal E, Barratt G. Nanotechnologies and controlled release systems for the delivery of antisense oligonucleotides and small interfering RNA. Br J Pharmacol. 2009;157:179–94.
Howard KA. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Adv Drug Deliv Rev. 2009;61:710–20.
Singha K, Namgung R, Kim WJ. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011;21:133–47.
Akhtar S, Hughes MD, Khan A, Bibby M, Hussain M, Nawaz Q, et al. The delivery of antisense therapeutics. Adv Drug Deliv Rev. 2000;44:3–21.
Akhtar S, Benter I. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–32.
Brown MD, Schatzlein AG, Uchegbu IF. Gene delivery with synthetic (non viral) carriers. Int J Pharm. 2001;229:1–21.
Fattal E, Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int J Pharm. 2008;364:237–48.
Hughes MD, Hussain M, Nawaz Q, Sayyed P, Akhtar S. The cellular delivery of antisense oligonucleotides and ribozymes. Drug Discov Today. 2001;6:303–15.
Jaaskelainen I, Urtti A. Cell membranes as barriers for the use of antisense therapeutic agents. Mini-Rev Med Chem. 2002;2:307–18.
Juliano R, Bauman J, Kang H, Ming X. Biological barriers to therapy with antisense and siRNA oligonucleotides. Mol Pharm. 2009;6:686–95.
Gao Y, Liu XL, Li XR. Research progress on siRNA delivery with nonviral carriers. Int J Nanomedicine. 2011;6:1017–25.
Endres T, Zheng M, Kilic A, Turowska A, Beck-Broichsitter M, Renz H, et al. Amphiphilic biodegradable PEG-PCL-PEI triblock copolymers for FRET-capable in vitro and in vivo delivery of siRNA and quantum dots. Mol Pharm. 2014;11:1273–81.
Weber N, Ortega P, Clemente MI, Shcharbin D, Bryszewska M, de la Mata FJ, et al. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J Control Release. 2008;132:55–64.
Zheng M, Pavan GM, Neeb M, Schaper AK, Danani A, Klebe G, et al. Targeting the blind spot of polycationic nanocarrier-based siRNA delivery. ACS Nano. 2012;6:9447–54.
Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotech. 2000;18:33–7.
Jimenez J, Clemente M, Weber N, Sanchez J, Ortega P, de la Mata F, et al. Carbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virus. BioDrugs. 2010;24:331–43.
Kim SS, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18:370–6.
Reynolds JL, Law WC, Mahajan SD, et al. Nanoparticle based galectin-1 gene silencing, implications inmethamphetamine regulation of HIV-1 infection in monocyte derived macrophages. J Neuroimmune Pharmacol. 2012;7(3):673–85.
Kuo YC, Kuo CY. Electromagnetic interference in the permeability of saquinavir across the blood brain barrier using nanoparticulate carriers. Int J Pharm. 2008;351(1–2):271–281.
Choi SU, Bui T, Ho RJ. pH dependent interactions of indinavir and lipids in nanoparticles and their ability to entrap a solute. J Pharm Sci. 2008;97(2):931–943.
Puligujja P, Balkundi SS, Kendrick LM, Baldridge HM, Hilaire JR, Bade AN, Dash PK, Zhang G, Poluektova LY, Gorantla S, Liu XM, Ying T, Feng Y, Wang Y, Dimitrov DS, McMillan JM, Gendelman HE. Pharmacodynamics of long acting folic acid receptor targeted ritonavirboosted atazanavir nanoformulations. Biomaterials. 2015;41:141–150.
Nowacek AS, McMillan J, Miller R, Anderson A, Rabinow B, Gendelman HE. Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. J Neuroimmune Pharmacol. 2010;5(4):592–601.
Prabhakar K, Afzal SM, Surender G, Kishann V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharmaceutica Sinica B. 2013;3(5):345–353.
Destache CJ, Belgum T, Christensen K, Shibata A, Sharma A, Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infectious Diseases. 2009;9:198. doi:10.1186/1471-2334-9-198.
Patil P, Sharma AK, Dadarwal SC, Sharma VK. Development of solid lipid nanoparticles of lamivudine for brain targeting. Int J Drug Deliv Tech. 2009;1(1):36–38.
Deastache CJ. Nanoparticles and methods of use. Patent Number US8846096 B2; 2014.
Chaubal MV, Kipp JE, Rabinow BE, Werling J. Nanosuspensions of anti-retroviral agents for Increased central nervous system delivery. Patent Number WO2005/072706 A3; 2005.
Boger JS, Chaturvedi PR, Tung RD. Nanosized aspartyl protease inhibitors. Patent Number WO1998047492 A1; 1998.
Destache C; Date A, Shibata A. Polymeric nanoparticle in a thermosensitive gel for coital independent vaginal prophylaxis of HIV. Patent Number US2015/0190398 A1; 2015.
Raveen Parboosing 1,*, Glenn E. M. Maguire 2, Patrick Govender 3 and Hendrik G. Kruger. Nanotechnology and the Treatment of HIV Infection. Viruses 2012; 4:488–520; doi:10.3390/v4040488.
Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3:242–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest. We gratefully acknowledge the Department of Science and Technology (DST), Government of India, for providing a research grant through DST-UKIERI scheme (DST/INT/UK/P-88/2014). Manuscript was written by M. K. D. and A. S., figures drafted by T. S., and manuscript edited by M. K. D. The authors declare no involvement of informed consent or animal studies.