Abstract
In 2016, necrosis symptoms was observed on stem and flower of red poppy (Papaver rhoeas) in Hamadan province of Iran. Symptomatic samples were collected and suspicious bacterial agent was isolated on nutrient agar medium. The phenotypic features of the bacterial strains were characterized and some molecular traits were examined. The bacterial strains phenotypically showed a high similarity to the members of Enterobacteriaceae, in particular Erwinia piriflorinigrans. All tested strains were pathogenic on red poppy and blossoms of pear under greenhouse condition. The phylogenetic analysis based on 16S rRNA and atpD genes sequences showed that representative strains were very similar to Erwinia piriflorinigrans. This is the first report of the presence of Erwinia piriflorinigrans as a causal agent of flower necrosis of red poppy worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papaver rhoeas L. (Papaveraceae) is an annual herb indigenous to Iran and many other regions in the world (zargari 1994). In Iranian folk medicine, the plant extract has been used for treatment of a wide range of diseases including inflammation, diarrhea, sleep disorders, treatment of cough, analgesia and also to reduce the withdrawal signs of opioid addiction (zargari 1994).
Erwinia species cause plant diseases that include mainly blights and wilts diseases. The pathogen usually starts to damage to the plant in the vascular tissue, then spreads throughout the plant. Ingress by the pathogen generally occurs through natural openings and wounds (Garrity et al. 2006).
The genus Erwinia includes pear pathogens such as Erwinia pyrifoliae, reported in Korea (Kim et al. 1999), Erwinia spp. causing bacterial shoot blight of pear (Tanii et al. 1981; Beer et al. 1995), E. uzenensis, the causal agent of bacterial black shoot disease of European pear in Japan (Mizuno et al. 2010; Matsuura et al. 2012), and the rosaceous epiphytes E. tasmaniensis (Geider et al. 2006) and E. billingiae (Mergaert et al. 1999).
Fire blight, caused by the invasive enterobacterium Erwinia amylovora, is a major disease threat to pome fruit production globally, for which it has profound socio-economic impact (Bonn and van der Zwet 2000). Epidemics can develop rapidly, resulting in scorched-like symptoms, with death of trees or entire orchards within a single season. Typical symptoms include flower necrosis, immature fruit rot, shoot recurvature, profuse bacterial ooze and cankers on woody tissues. The phytopathogen E. amylovora enters hosts through natural openings such as nectarthodes and wounds. The disease develops as blossom, shoot, trunk, or root stock blight depending of the plant tissue affected by the organism (Smits et al. 2013).
The species Erwinia piriflorinigrans, a Gram-negative plant-pathogenic bacterium was described to cause necrotic pear blossoms (López et al. 2011; Roselló et al. 2006). Unlike Erwinia amylovora, the causal agent of fire blight, E. piriflorinigrans affects blossoms and no other parts of plants (Roselló et al. 2006).
Red poppy is notable as an agricultural weed. In 2016, necrosis symptoms was observed on flower of red poppy in Hamadan province of Iran. The purpose of this study was to isolate the bacterium causing flower necrosis of red poppy in Hamadan province of Iran and also it’s identify using the analysis of several biochemical and molecular tests.
Materials and methods
Plant sampling and bacterial isolation
Affected flower samples of red poppy plants were collected from Hamadan province, they kept in paper bags at 4 °C for maximum three days to isolate the disease causative bacterium. Plant samples were washed under tap water, disinfected, washed again with sterilized distilled water, cut into small pieces and placed in sterilized distilled water for 30 min. A loop full of the suspensions, were streaked onto plates of nutrient agar (NA) and kept at 28 °C for 3 days. Single colonies were recovered and grown on the same media for the characterization of phenotypic features and they kept at 4 °C for further characterization (Schaad et al. 2001). The isolates were stored in 30% (v/v) glycerol solution at −80 °C for long term storage.
Pathogenicity test
Bacterial strains isolated from the plant tissues were grown on SNA for 24 h at 25 °C. They were suspended in sterilized distilled water and their concentration adjusted to 5 × 108 CFU/ml. The pathogenicity tests were performed by injection the bacterial suspension into poppy pods and stems using sterilized needles. Plants were covered with a plastic bag for 24 h to increase relative humidity and they were maintained in a greenhouse at 25 °C until symptoms appeared (Schaad et al. 2001). Eight representative strains were used in this experiment. The inoculated plants were monitored daily for symptoms development till 10 days after inoculation. The control plants were inoculated with sterile distilled water. Different bioassays (immature pear fruits, detached pear shoots, and detached pear blossoms) were carried out with representative strains as described by Roselló et al. (2006).
Phenotypic features
Phenotypic features of the bacterial strains were characterized based on standard bacteriological methods. These include; Gram staining: sensitivity to 3% KOH (Suslow et al. 1982), oxidative/fermentative test (Hugh and Leifson 1953) and Oxidase reaction (Kovacs 1956). Argenine dihydrolase, Levan formation, hydrolysis of gelatin, catalase production, nitrate reduction, flourescent production on King’s B medium and induce a hypersensitive reaction (HR) on geranium (Pelargonium × hortorum) were performed as described by Schaad et al. (2001). In addition, assimilation of carbon sources was tested using the basal medium of Ayers et al. (1919) supplemented with 0.1–0.3% of carbon compounds.
DNA preparation
Bacteria were grown on NA medium and kept at 28 °C for 24 h. Bacterial suspensions were prepared in sterile distilled water (108 CFU/ml) and were lysed by the addition of 1:10 volume of 10% KOH and heating the suspension at 100 °C for 5 min with subsequent cooling on ice. Lysates were centrifuged at 10000×g for 10 min, and the supernatants were used directly for PCR or stored at −20 °C until used. The quality of DNA was determined by electrophoresis on agarose gel (Ausubel et al. 1992).
Sequence analyses of 16S rRNA and atp D genes
The 16S rRNA PCR amplification of representative isolates was amplified using primers fD1 and rP2 (Weisburg et al. 1991); total volume reaction was 25 μl which contained 2.5 mM MgCl2, 0.2 mM dNTPs, 0.2 μM of each primer, 2.5 μl of 10X buffer (100 mM Tris-HCl, 500 mM KCl, pH 8.4), 1.25 U Taq DNA polymerase (CinnaGen, Iran) and 1 μl of template DNA. Amplification was performed in a TC-512 (Techne) Thermal Cycler with an initial denaturing temperature of 95 °C for 5 min, followed by 35 cycles of denaturation (94 °C, 1 min), annealing (59 °C, 1 min) and extension (72 °C, 2 min), with a final extension of 72 °C for 7 min.
atp D gene was amplified by using ATP D 01-F and ATP D 02-R primers (Brady et al. 2008). The amplification conditions included denaturation at 95 °C for 5 min, 3 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 2 min 15 s and elongation at 72 °C for 1 min 15 s, followed by 30 cycles of denaturation at 95 °C for 35 s, annealing at 55 °C for 1 min 15 s and elongation at 72 °C for 1 min 15 s and a further 7 min of elongation at 72 °C.
The amplified fragments were subjected to sequencing by BIONEER Co., South Korea. Sequences were edited in the BioEdit v. 7.0.5.2 (Hall 1999), they were compared with sequences obtained from GenBank. and aligned by Clustal W (Thompson et al. 1994). Phylogenetic trees were constructed in MEGA7 (Kumar et al. 2016) using the neighbor-joining (NJ) method (Saitou and Nei 1987) with the Kimura-2-parameter distance model (Kimura 1980). Bootstrap analyses were based on 1000 random resamplings. The sequence data were deposited in the National Center for Biotechnology Information (NCBI).
Results
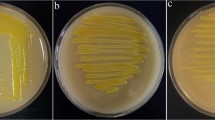
The symptoms observed on red poppy in 2016 affected the blossoms in spring (Fig. 1a). No symptoms were observed on pear trees in the sampling area. From infected red poppy plants, a total of 30 bacterial strains were isolated from difference region of Hamadan province (Table 1). They had smooth surfaces and entire margins, and were white, raised, transparent, and circular on NA medium. Tested bacterial strains were Gram negative, facultatively anaerobic, formed levan from sucrose, oxidase and arginine dihydrolase negative and catalase positive. Nitrates were not reduced and no fluorescent pigment were produced on King’s medium B after 48 h at 25 °C. They were urease-negative and caused hypersensitivity reaction on geranium. Table 2 showed phenotypic properties of the isolates.
Pathogenicity tests
The isolates induced flower necrosis of red poppy under greenhouse 3 days after inoculation (Fig. 1b). They were successfully inoculated on detached blossoms of pear and appeared after 3 to 4 days of incubation (Fig. 1c) but, they were not able to produce necrosis or ooze on immature fruitlets, and on detached shoots of pear. Re-isolations from the inoculated plants were done, and the isolated strain was checked by morphological characteristics and biochemical tests which confirms Koch’s postulates.
16S rDNA and atp D gene analyses
Blast analysis of the 16S rDNA sequence of the isolates YMA3 (GenBank accession number # KX852419) and YMA5 (GenBank accession number KX852421) shared 99% identity with Erwinia piriflorinigrans CFBP 5882. Phylogenetic analysis based on the 16S rRNA gene sequences showed that the representative isolates (YMA3 and YMA5) and reference strain Erwinia piriflorinigrans CFBP 5882 clustered in one group (Fig. 2).
The nucleotide sequences of 642 bp atpD gene were obtained for the selected isolates. PCR amplification and sequencing of the atpD gene of the isolates YMA3 and YMA5 yielded of a fragment sequence, approximately 642 bp long of atpD. The sequences of the isolates YMA3 and YMA5 (GenBank accession # KX852420 and KX852422) were 100% identical to the type strain of Erwinia piriflorinigrans CFBP 5882.Phylogenetic analysis based on atpD gene showed that representative isolates were phylogenetically close to Erwinia piriflorinigrans (Fig. 3).
Discussion
The characterized flower necrosis causal bacterium of red poppy were Gram-reaction-negative, facultatively anaerobic, oxidase negative, levan positive on SNA medium, nonfluorescent on King’s B medium, and without yellow coloneis on YDC medium. They were negative for nitrate reduction and arginine dihydrolase. Based on the determined phenotypic features they were belongs to Enterobacteriaceae, in particular the genus Erwinia (Schaad et al. 2001).
The biochemical and physiological traits of the tested strains were compared with Erwinia species. Results suggested that the strains of causal agent for flower necrosis of red poppy displayed the same phenotypic characteristics (Table 2) with Erwinia piriflorinigrans (Roselló et al. 2006; López et al. 2011).
The species Erwinia piriflorinigrans, a Gram-negative phytopathogenic bacterium, was described to cause necrotic pear blossoms (López et al. 2011). Unlike Erwinia amylovora, the causal agent of fire blight, E. piriflorinigrans attacks only blossoms and no other parts of plants are apparently affected (Roselló et al. 2006). Other usual fire blight hosts in the same area did not display any symptoms of infection with this Erwinia species (Smits et al. 2013).
Tested bacterial strains induced necrosis on red poppy and pear flowers under greenhouse 3 days after inoculation (Fig. 1b and c), they were not able to produce necrosis or forming ooze on immature fruits, and detached shoots of pear (Roselló et al. 2006). This finding suggests that red poppy can be an alternative plant host for E. piriflorinigrans but this bacterium were not isolated from symptoms observed on pear blossoms in sampling area. The abundance of red poppy plants in nature and presence of this bacterium on the plant blossoms may act as inoculum source potentially can cause outbreak pear blossoms necrosis.
Analysis of 16S rDNA sequences is a common tool for research in evolutionary development of organisms (Olsen et al. 1994). The complex structure of ribosomes does not allow significant sequence variation of rRNAs. By applying primers derived from consensus sequences, the 16S rDNA of bacteria is a suitable target for PCR amplification with these primers and for detection of nucleotide exchanges in the variable region within the genera (Weisburg et al. 1991). Two representative bacterial strains YMA3 and YMA5 16S rDNA sequence analyses showed 99% similarity to E. piriflorinigrans which is in agreement with the results of the phenotypic characteristics results (Fig. 2).
To obtain a higher resolution of the phylogenetic relationships of species within a genus or genera in the same family, multilocus sequence analyses (MLSA) is currently widely employed. In MLSA studies, partial sequences of genes coding for proteins with conserved functions (‘housekeeping genes’) are used to generate phylogenetic trees and subsequently deduce phylogenies (Glaeser and Kämpfer 2015). The sequences of housekeeping gene atpD of the YMA3 and YMA5 strains were also determined and compared which showed 100% similarity to E. piriflorinigrans (Fig. 3). Analyses of the sequences of these genes in tested bacterial strains showed that bacterium causing flower necrosis of red poppy is belongs to E. piriflorinigrans. This is the first report on the incidence of flower necrosis of red poppy caused by E. piriflorinigrans in Iran and worldwide.
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman J, Smith JA, Struhl K (1992) Short protocols in molecular biology
Ayers SH, Rupp P, Johnson WT (1919) A study of the alkali-forming bacteria found in milk. vol 782. US Department of Agriculture
Beer S, Kim J-H, Zumoff C, Bogdanove A, Laby R, Tanii A, Tamura O, Gustafson H, Momol T, Aldwinckle H (1995) Characterization of bacteria that cause" bacterial shoot blight of peard" in Japan. VII Int Workshop Fire Blight 411:179–182
Bonn W, van der Zwet T (2000) Distribution and economic importance of fire blight. In: Vanneste JL (ed) Fire Blight: The Disease and its Causative Agent, Erwinia amylovora. CABI Publishing. CABI Publishing, Wallingford, UK
Brady C, Cleenwerck I, Venter S, Vancanneyt M, Swings J, Coutinho T (2008) Phylogeny and identification of Pantoea species associated with plants, humans and the natural environment based on multilocus sequence analysis (MLSA). Syst Appl Microbiol 31(6):447–460
Garrity G, Staley JT, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H (2006) Bergey's Manual® of Systematic Bacteriology: Volume Two: The Proteobacteria. Springer Science & Business Media
Geider K, Auling G, Du Z, Jakovljevic V, Jock S, Völksch B (2006) Erwinia tasmaniensis sp. nov., a non-phytopathogenic bacterium from apple and pear trees. Int J Syst Evol Microbiol 56(12):2937–2943
Glaeser SP, Kämpfer P (2015) Multilocus sequence analysis (MLSA) in prokaryotic taxonomy. Syst Appl Microbiol 38(4):237–245
Hall TA BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series, 1999. vol 41. [London]: Information Retrieval Ltd., c1979-c2000., pp 95–98
Hugh R, Leifson E (1953) The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol 66(1):24
Kim W-S, Gardan L, Rhim S-L, Geider K (1999) Erwinia pyrifoliae sp. nov., a novel pathogen that affects Asian pear trees (Pyrus pyrifolia Nakai). Int J Syst Evol Microbiol 49(2):899–906
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120
Kovacs N (1956) Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature 178(4535):703–703
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular biology and evolution:msw054
López MM, Rosello M, Llop P, Ferrer S, Christen R, Gardan L (2011) Erwinia piriflorinigrans sp. nov., a novel pathogen that causes necrosis of pear blossoms. Int J Syst Evol Microbiol 61(3):561–567
Matsuura T, Mizuno A, Tsukamoto T, Shimizu Y, Saito N, Sato S, Kikuchi S, Uzuki T, Azegami K, Sawada H (2012) Erwinia uzenensis sp. nov., a novel pathogen that affects European pear trees (Pyrus communis L.) Int J Syst Evol Microbiol 62(8):1799–1803
Mergaert J, Hauben L, Cnockaert MC, Swings J (1999) Reclassification of non-pigmented Erwinia herbicola strains from trees as Erwinia billingiae sp. nov. Int J Syst Evol Microbiol 49(2):377–383
Mizuno A, Tsukamoto T, Shimizu Y, Ooya H, Matsuura T, Saito N, Sato S, Kikuchi S, Uzuki T, Azegami K (2010) Occurrence of bacterial black shoot disease of European pear in Yamagata Prefecture. J Gen Plant Pathol 76(1):43–51
Olsen GJ, Woese CR, Overbeek R (1994) The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol 176(1):1
Roselló M, Peñalver J, Llop P, Gorris M, Cambra M, López M, Chartier R, García F, Montón C (2006) Identification of an Erwinia sp. different from Erwinia amylovora and responsible for necrosis on pear blossoms. Can J Plant Pathol 28(1):30–41
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Schaad NW, Jones JB, Chun W (2001) Laboratory guide for the identification of plant pathogenic bacteria. vol Ed. 3. American Phytopathological Society (APS Press)
Smits TH, Rezzonico F, López MM, Blom J, Goesmann A, Frey JE, Duffy B (2013) Phylogenetic position and virulence apparatus of the pear flower necrosis pathogen Erwinia piriflorinigrans CFBP 5888 T as assessed by comparative genomics. Syst Appl Microbiol 36(7):449–456
Suslow T, Schroth M, Isaka M (1982) Application of a rapid method for Gram differentiation of plant pathogenic and saprophytic bacteria without staining. Phytopathology (USA)
Tanii A, Tamura O, Ozaki M (1981) Causal pathogen of fire blight-like symptoms of pear. Ann Phytopathol Soc Jpn 47:102
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Zargari A (1994) Medical Plants, vol 1. Tehran University, Tehran
Acknowledgements
The authors would like to thank the Research Deputy of the University of Bu-Ali Sina, Hamadan, Iran for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Moradi Amirabad, Y., Khodakaramian, G. Isolation and characterization of Erwinia piriflorinigrans causal agent flower necrosis of red poppy. Australasian Plant Pathol. 46, 611–616 (2017). https://doi.org/10.1007/s13313-017-0513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-017-0513-0