Abstract
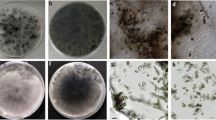
Members of the Botryosphaeriaceae commonly cause stem cankers and dieback of tropical and subtropical woody plants. A survey was conducted to isolate and identify the fungal causal agents of dieback and sooty canker of trees in some areas of Khuzestan province, southwestern Iran, 2015–2022. Following visual inspection, 63 trees belonging to 22 species were detected with typical signs of the disease in eight townships, and symptomatic material including branches and stems were sampled from those trees. Fungal isolation was performed on Potato Dextrose Agar (PDA), and the isolates obtained were induced into sporulation using pine needles in Water Agar (WA). All isolates were identified as Neoscytalidium novaehollandiae, based on multi-gene phylogenetic analyses of combined ITS, LSU, and tef-1α sequence data, in combination with morphological comparisons. In a pathogenicity trial, five representative strains of N. novaehollandiae were inoculated onto healthy stem fragments from their respective original host tree species, resulting in severe necrotic lesions in all cases. Field assessment of sooty canker and dieback of trees in areas under investigation showed that at least 5–30 percent of ornamental and fruit trees were affected by N. novaehollandiae, with disease severity of 11.1 to 28.2 percent. Ficus benghalensis, Morus nigra, Juglans regia and Eucalyptus camaldulensis trees were more frequently and severely affected, as compared to Cassia fistula and Syzygium cumini. This study reports 22 new hosts for N. novaehollandiae in association with serious disease symptoms in Iran.





Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov.
References
Kim KW, Lee IJ, Thoungchaleun V, Kim CS, Lee DK, Park EW (2009) Visualization of wound periderm and hyphal profiles in pine stems inoculated with the pitch canker fungus Fusarium circinatum. Microscopy Research and Technique 72:965–973
Ahmadpour SA, Mehrabi-Koushki M, Farokhinejad R (2017) Neodidymelliopsis farokhinejadii, a new fungal species from dead branches of trees in Iran. Sydowia 69:171–182
Akgül DS, Savaş NG, Özarslandan M (2019) First Report of Wood Canker Caused by Lasiodiplodia exigua and Neoscytalidium novaehollandiae on Grapevine in Turkey. Plant Disease 103:1036
Alizadeh M, Safaie N, Shams-Bakhsh M, Mehrabadi M (2022) Neoscytalidium novaehollandiae causes dieback on Pinus eldarica and its potential for infection of urban forest trees. Scientific Reports 12:9337
Alves A, Crous PW, Correia A, Phillips AJ (2008) Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity 28:1–13 http://www.fungaldiversity.org/fdp/sfdp/28-1.pdf
Anonym (2017) Thousand cankers disease survey guidelines for 2017. United States Department of Agriculture, Forest Service (FS) and Plant Protection and Quarantine (PPQ), USA
Arnold AE, Herre EA (2003) Canopy cover and leaf age affect colonization by tropical fungal endophytes: ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 95:388–398
Bakhshizadeh M, Hashemian HR, Najafzadeh MJ, Dolatabadi S, Zarrinfar H (2014) First report of rhinosinusitis caused by Neoscytalidium dimidiatum in Iran. Journal of Medical Microbiology 63:1017–1019
Berraf-Tebbal A, Mahamedi AE, Aigoun-Mouhous W, Špetík M, Čechová J, Pokluda R, Baranek M, Eichmeier A, Alves A (2020) Lasiodiplodia mitidjana sp. nov. and other Botryosphaeriaceae species causing branch canker and dieback of Citrus sinensis in Algeria. PLoS ONE 15:e0232448
Botella L, Santamaría O, Diez J (2010) Fungi associated with the decline of Pinus halepensis in Spain. Fungal Diversity 40:1–11
Brito AC, De Mello JF, Câmara MP, Vieira JC, Michereff SJ, Souza-Motta CM, Machado AR (2020) Diversity and pathogenicity of Botryosphaeriaceae species associated with black root rot and stem cutting dry rot in Manihot esculenta in Brazil. European Journal of Plant Pathology 157:583–598
Calavan EC, Wallace JM (1954) Hendersonula toruloidea Natrass on citrus in California. Phytopathology 44:635–639
Cao ZM, Wang X (1989) A survey of stem diseases of Zanthoxylun. Forest Pest Disease 1:47
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WF, Philips AJ, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55:235–253
Calvillo-Medina RP, Martínez-Neria M, Mena-Portales J, Barba-Escoto L, Raymundo T, Campos-Guillén J, Jones GH, Reyes-Grajeda JP, González-y-Merchand JA, Bautista-de Lucio VM (2018) Identification and biofilm development by a new fungal keratitis aetiologic agent. Mycoses 62:62–72
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9:772–772
Delgado-Cerrone L, Mondino-Hintz P, Alaniz-Ferro S (2016) Botryosphaeriaceae species associated with stem canker, die-back and fruit rot on apple in Uruguay. European Journal of Plant Pathology 146:637–655
Derviş S, Güney İG, Koşar İ, Bozoğlu T, Özer G (2021) First report of Neoscytalidium novaehollandiae on common sage (Salvia ofcinalis). Australasian Plant Disease Notes 16:1–4
Derviş S, Özer G, Türkölmez Ş (2020) First report of Neoscytalidium novaehollandiae causing stem blight on tomato in Turkey. Journal of Plant Pathology 102:1339–1340
Desprez-Loustau ML, Robin C, Reynaud G, Déqué M, Badeau V, Piou D, Husson C, Marçais B (2007) Simulating the effects of a climate-change scenario on the geographical range and activity of forest-pathogenic fungi. Canadian Journal of Plant Pathology 29:101–120
Dionne B, Neff L, Lee SA, Sutton DA, Wiederhold NP, Lindner J, Fan H, Jakeman B (2015) Pulmonary fungal infection caused by Neoscytalidium dimidiatum. Journal of Clinical Microbiology 53:2381–2384
Dissanayake AJ, Zhang W, Li X, Zhou Y, Chethana T, Chukeatirote E, Hyde KD, Yan J, Zhang G, Zhao W (2015) First report of Neofusicoccum mangiferae associated with grapevine dieback in China. Phytopathologia Mediterranea 54:414–419
Edler D, Klein J, Antonelli A, Silvestro D (2019) raxmlGUI 2.0 beta: a graphical interface and toolkit for phylogenetic analyses using RAxMl. bioRxiv, https://doi.org/10.1101/800912
Elliott M, Edmonds RL, Suff LJ (1997) Role of stress in development of Arbutus canker (Nattrassia mangiferae). Phytopathology 87:S27
Elshafie AE, Ba-Omar T (2002) First report of Albizia lebbeck dieback caused by Scytalidium dimidiatum in Oman. Mycopathologia 154(1):37–40
Ershad J (2009) Fungi of Iran. Iranian Research, Educations Institute of plant protection, Iran, Tehran, p 531
Fan XL, Du Z, Hyde KD, Liang YM, PAN YP, Tian CM (2016) Cryptosporella platyphylla, a new species associated with Betula platyphylla in China Phytotaxa 253:285–292
Giha OH (1975) Hendersonula toruloidea associated with a serious wilt disease of shade trees [Fiscus benghalensis] in the Sudan [Fungus diseases]. Plant Disease Reporter 59:899–902
Goudarzi A, Moslehi M (2020) Distribution of a devastating fungal pathogen in mangrove forests of southern Iran. Crop Protection 128:104987
Granata G, Sidoti A (1991) Grapevine death caused by Nattrassia toruloidea. Vitis 30:219–222
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/ NT. Nucleic Acids Symposium Series 41:95–98
Harsh NSK, Tiwari CK (1992) Top dying and mortality in provenence trial of Gemelina arborea. Journal of Tropical Forest 8(1):55–61
Heidarian A, Alizadeh A (1995) Citrus dieback and decline caused by Nattrassia mangiferae and its other hosts in Khouzestan province. In Proceedings of the 12th Iranian Plant Protection Congress 2–7 September 1995, Karaj, Iran, pp 231
Horst RK (2013) Field manual of diseases on trees and shrubs (p. 196). Springer, Dordrecht
Huang SK, Tangthirasunun N, Phillips AJ, Dai DQ, Wanasinghe DN, Wen TC, Bahkali AH, Hyde KD, Kang JC (2016) Morphology and Phylogeny of Neoscytalidium orchidacearum sp. nov. (Botryosphaeriaceae). Mycobiology 44:79–84
Kazemzadeh Chakusary M, Mohammadi H, Khodaparast SA (2019) Diversity and pathogenicity of Botryosphaeriaceae species on forest trees in the north of Iran. European Journal of Forest Research 15:685–704
Kornerup A, Wanscher JH (1967) Methuen handbook of colour. England, London
Kurt Ş, Uysal A, Soylu EM, Kara M, Soylu S (2019) First record of Neoscytalidium novaehollandiae associated with pistachio dieback in the Southeastern Anatolia region of Turkey. Mycologia Iranica 6:55–57
Madrid H, Ruíz-Cendoya M, Cano J, Stchigel A, Orofino R, Guarro J (2009) Genotyping and in vitro antifungal susceptibility of Neoscytalidium dimidiatum isolates from different origins. International Journal of Antimicrobial Agents 34:351–354
Manawasinghe IS, Phillips AJ, Hyde KD, Chethana KW, Zhang W, Zhao WS, Yan JY, Li XH (2016) Mycosphere Essays 14: Assessing the aggressiveness of plant pathogenic Botryosphaeriaceae. Mycosphere 7:883–892
Matheron ME, Sigler L (1993) First report of Eucalyptus die-back cause by Nattrassia mangiferae in North America. Plant Disease 78:432
Mayorquin JS, Wang DH, Twizeyimana M, Eskalen A (2016) Identification, distribution, and pathogenicity of Diatrypaceae and Botryosphaeriaceae associated with citrus branch canker in the southern California desert. Plant Disease 100:2402–2413
Mehrabi-Koushki M, Khodadadi-Pourarpanahi S, Jounbozorgi S (2018) Fungal endophytes associated with some thermotolerant plants in salt-stress ecosystem. Mikologiya i Fitopatologiya 52:187–195
Meredith DS (1963) Tip rot of banana fruits in Jamaica. I. Hendersonula toruloidea on Dwarf Cavendish bananas. Transactions of the British Mycological Society 46:473–481
Msikita W, Yaninek JS, Ahounou M, Baimey H, Fagbemissi R (1997) First report of Nattrassia mangiferae root and stem rot of cassava in West Africa. Plant Disease 81:332
Namsi A, Zouba A, Triki MA, Mahmoud MO, Takrouni ML (2010) Study on Nattrassia mangiferae, the causal agent of apricot tree decline disease in the Oases of South Tunisia: biology and in vitro evaluation of some fungicides. The African Journal of Plant Science and Biotechnology 4:88–90
Natour RM, El-Haideri HAIDER (1967) Occurrence of branch wilt disease caused by Hendersonula toruloidea in Iraq. Plant Disease Reporter 51:371–373
Nattrass RM (1933) A new species of Hendersonula toruloideae on deciduous trees in Egypt. Transactions of the British Mycological Society 18:189–198
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences 95:2044–2049
O’Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 225–233
Oksal E, Özer G (2021) First report of shoot blight and branch canker of Pyrus communis by Neoscytalidium novaehollandiae in Turkey. Journal of Plant Pathology 103:673–674
Ören E, Koca G, Bayraktar H (2020a) First report of Neoscytalidium novaehollandiae associated with branch dieback on Japanese persimmon in Turkey. Journal of Plant Pathology 102:1311–1312
Ören E, Koca G, Gencer R, Bayraktar H (2020b) First report of Neoscytalidium novaehollandiae associated with stem canker and branch dieback of almond trees Australasian Plant Disease. Notes 15:1–3
Pavlic D, Wingfield MJ, Barber P, Slippers B, Hardy GE, Burgess TI (2008) Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100:851–866
Phillips AJ, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 55:53–63
Raeder U, Broda P (1985) Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology 1:17–20
Rahmani S (2018) Morphological and molecular identifcation of Neoscytalidium dimidiatum isolates and other endophyte fungi dieback in Sistan and Kerman Province. Doctoral dissertation, University of Zabol
Ray JD, Burgess T, Lanoiselet V (2010) First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australasian Plant Disease Notes 5:48–50
Reckhans P, Adamou I (1987) Hendersonula dieback of Mango in Niger. Plant Disease 71:1045
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542
Ruíz-Cendoya M, Madrid H, Pastor FJ, Mayayo E, Mariné M, Guarro J (2010) Development of murine models of disseminated infection by Neoscytalidium dimidiatum. Medical Mycology 48:681–686
Sabernasab M, Jamali S, Marefat A, Abbasi S (2019) Morphological and molecular characterization of Neoscytalidium novaehollandiae, the cause of Quercus brantii dieback in Iran. Phytopathologia Mediterranea 58:347–357
Sakalidis ML, Ray JD, Lanoiselet V, Hardy GE, Burgess TI (2011) Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley region of Western Australia. European Journal of Plant Pathology 130:379–391
Shokoohi GR, Ansari S, Abolghazi A, Gramishoar M, Nouripour-Sisakht S, Mirhendi H, Makimura K (2019) The First Case of Fingernail Onychomycosis Due to Neoscytalidium novaehollandiae, Molecular Identification and Antifungal Susceptibility. Journal De Mycologie Medicale 30:100920
Shurtleff MC (1997) Deter canker and dieback diseases. Grounds Maintenance 32:49–56
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biology Reviews 21:90–106
Smith H, Wingfield MJ, Petrini O (1996) Botryosphaeria dothidea endophytic in Eucalyptus grandis and Eucalyptus nitens in South Africa. Forest Ecology and Management 89:189–195
Sommer NF (1955) Sunburn predisposes walnut trees to branch wilt. Phytopathology 45:607–613
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
Tsahouridou PC, Thanassoulopoulos CC (2000) First report of Hendersonula toruloidea as a foliar pathogen of strawberry-tree (Arbutus unedo) in Europe. Plant Disease 84:487
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Whiteside JO (1988) Miscellaneous fungi and associated diseases. In: Whiteside JO, Garnsey SM, Timmer LW (eds) Compendium of Citrus Diseases. The American Phytopathological Society, Minnesota, USA, p 29
Wonglom P, Pornsuriya C, Sunpapao A (2023) A New Species of Neoscytalidium hylocereum sp. nov. Causing Canker on Red-Fleshed Dragon Fruit (Hylocereus polyrhizus) in Southern Thailand. Journal of Fungi 3:197
Zhang W, Groenewald JZ, Lombard L, Schumacher RK, Phillips AJ, Crous PW (2021) Evaluating species in Botryosphaeriales. Persoonia-Molecular Phylogeny and Evolution of Fungi 46:63–115
Zhu XM, Liu XF (2012) A new species and genus distribution record from China: Neoscytalidium novaehollandiae. Indian Journal of Microbiology 52:565–568aaa
Acknowledgements
Authors would like to appreciate the administration of plant protection Department, Faculty of Agriculture, Shahid Chamran University of Ahvaz for their support in accomplishment of this study. The authors are thankful to anonymous reviewers for beneficial comments and suggestions on the manuscript.
Funding
This work was financially supported by grant (SCU.AP1401.294) from Research Council of Shahid Chamran University of Ahvaz.
Author information
Authors and Affiliations
Contributions
Seyedeh Akram Ahmadpour carried out sample preparation, fungal isolation and purification, morphometric and morphological determination, DNA isolation and amplification, DNA and phylogenetic analysis and the writing of the manuscript. Mehdi Mehrabi-Koushki carried out the design and implementation of the research and revising of the manuscript. Reza Farokhinejad contributed to the implementation of the research and revising of the manuscript. Zahra Mirsoleymani, provided some fungal isolates and revising of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmadpour, S.A., Mehrabi-Koushki, M., Farokhinejad, R. et al. Characterization and pathogenicity of Neoscytalidium novaehollandiae causing dieback and sooty canker in Iran. Trop. plant pathol. 48, 493–507 (2023). https://doi.org/10.1007/s40858-023-00591-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-023-00591-8