Abstract
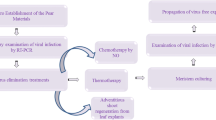
Four varieties of potted apple were subjected to thermotherapy (37 ± 1 °C) coupled with shoot tip grafting. Using reverse transcription-polymerase chain reaction assays prior to treatment, mixed infections with Apple chlorotic leaf spot virus (ACLSV), Apple stem pitting virus (ASPV), Apple stem grooving virus (ASGV), Apple mosaic virus (ApMV) and Apple scar skin viroid (ASSVd) were detected in 95.8 % of treated plants. It was found that the sprouts of Dahongrong (DHR) and Qiyuetianxian (QYT) variety buds were inhibited by high temperature, although some buds of these two varieties were able to sprout, the growth was slow. The average survival rate of shoot tips cut after thermotherapy was 41.2 % (61/148). The survival rate of DHR (63.2 %) was highest among the four varieties. Sixty-one surviving apple plants were detected over two periods, indicative of an average elimination rate of 65.6 % (40/61) for new leaves (June) and 37.7 % (23/61) for dormant branches (November). The difference of elimination rate for DHR between the two periods was the most obvious. Detections rates of viruses were also different in the two periods; ASGV in dormant branches were 27.8 % higher than those in new leaves, and ASPV (90.6 %) remained the same over the two periods.


Similar content being viewed by others
References
Bhardwaj SV, Rai SJ, Thakur PD, Handa A (1998) Meristem tip culture and heat therapy for production of Apple mosaic virus free plants in India. Acta Hortic 472:65–68
Campbell AI (1963) The effect of some latent virus infections on the growth and cropping of apples. J Hort Sci 38:15–19
Campell AI (1968) Heat sensitivity of some apple viruses. Tagungsber DAL DDR (Berlin) 97:311–316
Chellappan P, Vanitharani R, Ogbe F, Fauquet CM (2005) Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol 138:1828–1841
Cieslinska M, Malinowski T, Zawadzka BJ (1995) Studies on several strains of Apple chlorotic leaf spot virus (ACLSV) isolated from different fruit tree species. Acta Hortic 386:63–71
Clover GRG, Pearson MN, Elliott DR, Tang Z, Smales TE, Alexander BJR (2003) Characterization of a strain of Apple stem grooving virus in Actinidia chinensis from China. Plant Pathol 52:371–378
Cordewener JHG, Hause G, Gorgen E, Busink R, Hause B, Dons HJM, Van Lamen AMA, Van Lookeren Campagne MM, Pechan P (1995) Changes in synthesis and localisation of the 70-kDa class of heat-shock proteins accompany the induction of embryogenesis in Brassica napus L. microspores. Planta 196:747–755
Da Câmara Machado A,Mendonça D, Lopes MS, Knapp E, Hanzer V, Arthofer W, Katinger H, Laimer de Câmara Machado M (1998) Phytosanitary improvement of fruit tree species: diagnostic strategies in virus-indexing of in vitro plants. Acta Hortic 472:511–516
Desvignes JC (1999) Virus diseases of fruit trees. Certification schemes-pathogen-tested material of Malus, Pyrus and Cydonia. EPPO Bull. Ctifl Paris 29:239–252
Desvignes JC, Grasseau N, Boye R, Cornaggia D, Aparicio F, Di Serio F, Flores R (1999) Biological properties of Apple scar skin viroid: Isolates, host range, different sensitivity of apple cultivars, elimination, and natural transmission. Plant Dis 83:768–772
Foissac X, Svanella-Dumas L, Dulucq MJ, Candresse T, Gentit P (2001) Polyvalent detection of fruit tree tricho, capillo and foveaviruses by nested RT-PCR using degenerated and inosine containing primers (PDO RT-PCR). Acta Hortic 1:37–44
Hansen AJ (1989) Antiviral chemicals for plant disease control. Crit Rev Plant Sci 8:85–88
Hoffman MT, Doud MS, Williams L, Zhang MQ, Ding F, Stover E, Hall D, Zhang S, Jones L, Gooch M, Fleites L, Dixon W, Gabriel D, Duan YP (2013) Heat treatment eliminates ‘Candidatus Liberibacter asiaticus’ from infected citrus trees under controlled conditions. Phytopathology 103:15–22
Hoss M, Paabo S (1993) DNA extraction from pleistocene bones by a silica-based purification method. Nucleic Acids Res 21:3913–3914
Hu GJ, Hong N, Wang LP, Hu HJ, Wang GP (2012) Efficacy of virus elimination from in vitro-cultured sand pear (Pyrus pyrifolia) by chemotherapy combined with thermotherapy. Crop Prot 37:20–25
James D, Trytten P, MacKenzie DJ, Towers GHN, French CJ (1997) Elimination of Apple stem grooving virus by chemotherapy and development of an immunocapture RT-PCR for rapid sensitive screening. Ann Appl Biol 131:459–470
Kassanis B (1957) Effects of changing temperature on plant virus diseases. Adv Virus Res 4:221–241
Knapp E, Hanzer V, Weiss H, Da Câmara MA, Weiss B, Wang Q, Katinger H, da Câmara L, Machado M (1995) New aspects of virus elimination in fruit trees. Acta Hort 386:409–418
Kudela V, Krejzar V, Kundu JK, Pankova I, Ackermann P (2009) Apple burrknots involved in trunk canker initiation and dying of young trees. Plant Prot Sci 45:1–11
Liu FC, Wang HY (1989) On latent viruses of apple in China II: indexing of latent viruses in apple scion varieties and dwarf rootstocks. Acta Phytopathol Sinica 19:193–197
Ma BG, Niu JX, Morley-Bunker M, Pan LZ, Zhang HP, Zhang LX (2008) Detection of three pear viruses by multiplex RT-PCR assays with co-amplification of an internal control. Australas Plant Pathol 37:117–122
Maliogka VI, Skiada FG, Eleftheriou EP, Katis NI (2009) Elimination of a new ampelovirus (GLRaV-Pr) and Grapevine rupestris stem pitting associated virus (GRSPaV) from two Vitis vinifera cultivars combining in vitro thermotherapy with shoot tip culture. Sci Hortic 123:280–282
Manganaris GA, Economou AS, Boubourakas IN, Katis NI (2003) Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep 22:195–200
Menzel W, Jelkmann W, Maiss E (2002) Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. J Virol Methods 99:81–92
Mink GI, Wample R, Howell WE (1998) Heat treatment of perennial plants to eliminate phytoplasmas, viruses, and viroids while maintaining plant survival. In: Hadidi A, Khetarpal RK, Koganezawa H (eds) Plant virus disease control. APS Press, St. Paul, MN, USA, pp 332–345
Panattoni A, Triolo E (2010) Susceptibility of grapevine viruses to thermotherapy on in vitro collection of Kober 5BB. Sci Hortic 125:63–67
Paprstein F, Sedlak J, Polak J, Svobodova L, Hassan M, Bryxiova M (2008) Results of in vitro thermotherapy of apple cultivars. Plant Cell Tiss Organ Cult 94:347–352
Paunović S, Jevremović D (2006) Elimination of viruses from the infected apple cv Fuji Naga-Fu 2 by thermotherapy. Voćarstvo 40:189–197
Penrose LJ, Davis KC, Valentine BJ (1988) Comparative performance of three apple clones derived from a virus-tested scheme, with clones infected with latent viruses and a mycoplasma. Sci Hortic 36:55–65
Salem N, Mansour A, Al-Musa A, Al-Nsour A, Hammond R (2004) Identification and partial characterization of Prunus necrotic ringspot virus on stone fruits in Jordan. J Plant Pathol 86:85–90
Schmidt H (1972) The effect of ‘latent’ virus infections on the yield of maiden trees on 20 apomictic apple seedling root-stocks. J Hort Sci 47:159–163
Sedlák J, Paprštein F, Svobodová L, Talácko L (2013) In vitro thermotherapy and chemotherapy of apple cultivar ‘Jarka’. Vědecké Práce Ovocnářské 23:233–240
Sharma S, Singh B, Rani G, Zaidi AA, Hallan VK, Nagpal AK, Virk GS (2008) In vitro production of Indian citrus ringspot virus (ICRSV) free kinnow plants employing phytotherapy coupled with shoot tip grafting. Plant Cell Tiss Organ Cult 92:85–92
Sipahioglu HM, Usta M, Ocakm M (2006) Use of dried high-phenolic laden host leaves for virus and viroid preservation and detection by PCR methods. J Virol Methods 137:120–124
Tan RR, Wang LP, Hong N, Wang GP (2010) Enhanced efficiency of virus eradication following thermotherapy of shoot-tip cultures of pear. Plant Cell Tiss Org Cult 101:229–235
Valero M, Ibanez A, Morte A (2003) Effects of high vineyard temperatures on the grapevine leaf-roll associated virus elimination from Vitis vinifera L. cv. Napoleon tissue cultures. Sci Hortic 97:289–296
Walia Y, Dhir S, Ram R, Zaidi AA, Hallan V (2014) Identification of the herbaceous host range of Apple scar skin viroid and analysis of its progeny variants. Plant Pathol 63:684–690
Wang L, Wang G, Hong N, Tang R, Deng X, Zhang H (2006) Effect of thermotherapy on elimination of Apple stem grooving virus and Apple chlorotic leaf spot virus for in vitro-cultured pear shoot tips. HortSci 41:729–732
Wang Q, Cuellar WJ, Rajamaki ML, Hirata Y, Valkonen JPT (2008) Combined thermotherapy and cryotherapy for efficient virus eradication: relation of virus distribution, subcellular changes, cell survival and RNA degradation in shoot tips. Mol Plant Pathol 9:237–250
Wang LP, Hong N, Wang GP, Xu WX, Michelutti R, Wang AM (2010) Distribution of Apple stem grooving virus and Apple chlorotic leaf spot virus in infected in vitro pear shoots. Crop Prot 29:1447–1451
Welsh MF, Nyland G (1965) Elimination and separation of viruses in apple clones by exposure to dry heat. Can J Plant Sci 45:443–454
Zhang ZP, Hong N, Zhang SY (1996) Improvement of virus elimination of apple by thermotherapy. North Fruits 4:12–13
Zilka S, Faingersh E, Rotbaum A, Tam Y, Spiegel S, Malca N (2002) In vitro production of virus-free pear plants. Acta Hortic 596:77–479
Acknowledgments
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (201203076) and Youth Foundation of Research Institute of Pomology, Chinese Academy of Agriculture Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, GJ., Zhang, ZP., Dong, YF. et al. Efficiency of virus elimination from potted apple plants by thermotherapy coupled with shoot-tip grafting. Australasian Plant Pathol. 44, 167–173 (2015). https://doi.org/10.1007/s13313-014-0334-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-014-0334-3