Abstract
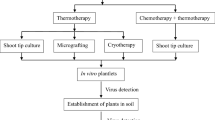
In this study, in vitro-cultured plants of Pyrus pyrifolia cv. Fengshui, widely grown in central and southern China, were treated at different temperatures, and the efficiency of virus eradication of regenerated plants was evaluated by ELISA and dot-blot hybridization. Results showed that treatment at a constant high temperature of 37°C influenced greatly on the growth of in vitro pear plants and significantly decreased the plant survival rate, though virus-free plants could be regenerated from a few survived plant tips. When the treatment was carried out at 32°C/night and 38°C/day, the survival rate and growth of pear plants could be significant improved, but no virus-free plants could be regenerated from shoot tips treated for 50 days. Based on these results, infected plants were heat-treated at 42°C/day and 34°C/night. High virus eradication efficiency was achieved from treated cultures of pear for more than 55 days. Results also verified that ACLSV could be eliminated from pear by 5 days shorter treatment period than that for ASGV and ASPV eradication.

Similar content being viewed by others
References
Adams MJ, Antoniw JF, Bar-Joseph M, Brunt AA, Candresse T, Foster GD, Martelli GP, Milne RG, Zavriev SK, Fauquet CM (2004) The new plant virus family Flexiviridae and assessment of molecular criteria for species demarcation. Arch Virol 149:1045–1060
Campell AI (1968) Heat sensitivity of some apple viruses. Tagungsber. DAL DDR (Berlin) 97:311–316
Chellappan P, Vanitharani R, Ogbe F, Fauquet CM (2005) Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol 138:1828–1841
Cieślińska M (2002) Elimination of Apple chlorotic leaf spot virus (ACLSV) from pear by in vitro thermotherapy and chemotherapy. Acta Hortic 596:481–484
Clover GRG, Pearson MN, Elliott DR, Tang Z, Smales TE, Alexander BJR (2003) Characterization of a strain of apple stem grooving virus in Actinidia chinensis from China. Plant Pathol 52:371–378
Cooper VC, Walkey DGA (1978) Thermal inactivation of cherry leaf roll virus in tissue cultures of Nicotiana rustica raised from seeds and meristem tips. Ann Appl Biol 88:273–278
Desvignes JC, Boye R (1989) Different diseases caused by the Apple chlorotic leaf spot virus on the fruit trees. Acta Hortic 235:31–38
Faccioli VC, Marani F (1998) Virus elimination by meristem tip culture and tip micrografting. In: Hadidi A, Khetarpal RK, Koganezawa H (eds) Plant Virus Disease Control. APS Press, St. Paul, MN, USA, pp 346–380
Foster TM, Lough TJ, Emerson SJ, Lee RH, Bowman JL, Forster RLS, Lucas WJ (2002) A surveillance system regulates selective entry of RNA into the shoot apex. Plant Cell 14:1497–1508
Hong N, Wang GP (1999) The biological and biochemical characterization of Apple chlorotic leaf spot virus. Acta Phytopathologica Sinica 29:77–81
Hong N, Wang GP, Yu JM, Dong YF, Zhang ZP (1997) The isolation, purification and serological detection of Apple stem grooving virus. Scientia Agricutura Sinica 30:6–12
Kassanis B (1957) Effects of changing temperature on plant virus diseases. Adv Virus Res 4:221–241
Knapp E, Hanzer V, Weiss H, da Câmara Machado A, da Clmara Machado A, Weiss B, Wang Q, Katinger H, Laimer da Clmara Machado M (1995) New aspects of virus elimination in fruit trees. Acta Hortic 386:409–418
Manganaris GA, Economou AS, Boubourakas IN, Katis NI (2003) Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep 22:195–200
Mink GI, Wample R, Howell WE (1998) Heat treatment of perennial plants to eliminate phytoplasmas, viruses, and viroids while maintaining plant survival. In: Hadidi A, Khetarpal RK, Koganezawa H (eds) Plant Virus Disease Control. APS Press, St. Paul, MN, USA, pp 332–345
Mochizuki T, Ohki ST (2004) Shoot meristem tissue of tobacco inoculated with cucumber mosaic virus is infected with the virus and subsequently recovers from infection by RNA silencing. J Gen Plant Pathol 70:363–366
Nemeth M (1986) Virus, mycoplasma and richettsia diseases of fruit trees. Martinus Nijhoff/Dr W. Junk Publishers, Dordrecht, Boston, Lancaster
Paprstein F, Sedlak J, Polak J, Svobodova L, Hassan M, Bryxiova M (2008) Results of in vitro thermotherapy of apple cultivars. Plant Cell Tiss Organ Cult 94:347–352
Plese N, Hoxha E, Milicic D (1975) Pathological anatomy of trees affected with apple stem grooving virus. Phytopathol 82:315–325
Qu F, Ye XH, Hou GC, Sato S, Clemente TE, Morris TJ (2005) RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J Virol 79:15209–15217
Quak F (1987) Therapy of individual plants. In: De Bokx JA, van der Want JPH (eds) Viruses of potatoes and seed-potato production. Pudoc, Wageningen, The Netherlands, pp 151–161
Schwach F, Vaistij FE, Jones L, Baulcombe DC (2005) An RNA dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol 138:1842–1852
Sharma S, Singh B, Rani G, Zaidi AA, Hallan VK, Nagpal AK, Virk GS (2008) In vitro production of Indian citrus ringspot virus (ICRSV) free kinnow plants employing phytotherapy coupled with shoot tip grafting. Plant Cell Tiss Organ Cult 92:85–92
Spiegel S, Stein A, Tam Y (1995) In vitro thermotherapy of Rosaceous fruit trees. Acta Hortic 386:419–420
Stein A, Spiegel S, Faingersh G, Levy S (1991) Responses of micropropagated peach cultivars to thermotherapy for the elimination of Prunus necrotic ringspot virus. Ann Appl Biol 119:265–271
Szittya G, Silhavy D, Molnar A, Havelda Z, Lovas A, Lakatos L, Banfalvi Z, Burgyan J (2003) Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. The EMBO J 22:633–640
Verma N, Ram R, Zaidi AA (2005) In vitro production of Prunus necrotic ringspot virus-free begonias through chemo- and thermotherapy. Scientia Hortic 103:239–247
Walkey DGA, Cooper VC (1975) Effect of temperature on virus eradication and growth of infected tissue cultures. Ann Appl Biol 80:185–190
Wang LP, Wang GP, Hong N, Tan RR, Deng XY, Zhang H (2006a) Effect of thermotherapy on elimination of Apple stem grooving virus and Apple chlorotic leaf spot virus for in vitro-cultured pear shoot tips. HortScience 41:729–732
Wang LP, Wang GP, Tan RR, Hong N (2006b) Detection of three latent viruses in pear by hybridization with biotinylated cDNA probes. Acta Phytopathologica Sinica 36:488–493
Wang QC, Cuellar WJ, RajamÄki ML, Hirata Y, Valkonen JPT (2008) Combined thermotherapy and cryotherapy for efficient virus eradication: relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol Plant Pathol 9:237–250
Wang QC, Panis B, Engelmann F, Lambardi M, Valkonen JPT (2009) Cryotherapy of shoot tips: a technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Ann Appl Biol 154:351–363
Yanase H (1983) Back transmission of Apple stem grooving virus to apple seedlings and induction of symptoms of apple topworking disease in Mitsuba Kaido (Malus sieboldii) and Kobano Zumi (Malus sieboldii var. arborescens) rootstocks. Acta Hortic 130:117–122
Zheng YY, Wang GP, Hong N (2005) The biological characteristics and molecular identification of some apple stem grooving virus isolates. Acta Phytophylacica Sinica 32:266–270
Zilka S, Faingersh E, Rotbaum A, Tam Y, Spiegel S, Malca N (2002) In vitro production of virus-free pear plants. Acta Hortic 596:477–479
Acknowledgments
This study was financially supported by National Natural Science Foundation of China (grant no: 30370997) and the earmarked fund for Pear Modern Agro-industry Technology Research System. The authors would like to thank Professor Dr. Ch. X. Luo, Huazhong Agricultural University and X. D. Li, Shandong Agricultural University, for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, R., Wang, L., Hong, N. et al. Enhanced efficiency of virus eradication following thermotherapy of shoot-tip cultures of pear. Plant Cell Tiss Organ Cult 101, 229–235 (2010). https://doi.org/10.1007/s11240-010-9681-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9681-0