Abstract
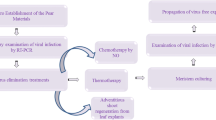
The plum pox virus (PPV) and prunus necrotic ringspot virus (PNRSV) cause serious disease problems in stone-fruit trees. In this work, the possibility of obtaining plant material free from these viruses through thermotherapy and meristem-tip culture from infected nectarine shoots (Prunus persica var. nectarina Max, cv. 'Arm King') was studied. In addition, the detection of these viruses in in vitro cultures and young acclimatized plantlets with double antibody sandwich-enzyme-linked immunosorbent assay (DAS-ELISA) and multiplex reverse transcriptase-polymerase chain reaction (RT-PCR) was studied. Meristem-tip explants (0.8–1.3 mm) derived from sprouted buds of winter wood and spring shoots from field grown plants had a 2–5% regeneration response. However, application of thermotherapy to potted nectarine trees (3 weeks at a maximum temperature of 35°C) facilitated excision of longer meristem tips (1.3–2.0 mm) that resulted in a significantly higher regeneration response (38%) in woody plant medium (WPM) without plant growth regulators. Such explants formed multiple shoots with the addition of 8 μM benzylaminopurine and 0.8 μM indoleacetic acid. When they were tested for the presence of PPV and PNRSV, 86% and 81% were found to be virus-free as detected by DAS-ELISA and multiplex RT-PCR, respectively. Individual shoots excised from virus-free cultures readily rooted in vitro (half-strength WPM plus 2 μM indolebutyric acid) and grew to plantlets. The combination of an efficient protocol for virus elimination and the establishment of highly sensitive diagnostics resulted in the production of nectarine plants free from PPV and PNRSV.




Similar content being viewed by others
Abbreviations
- cDNA :
-
Complementary DNA
- DAS-ELISA :
-
Double antibody sandwich-ELISA
- DSMZ :
-
Deutsche Sammlung von Mikroorganismen und Zelkulturen
- ELISA :
-
Enzyme-linked immunosorbent assay
- IC-RT-PCR :
-
Immunocapture-RT-PCT
- IgG :
-
Immunoglobulin G
- OD :
-
Optical density
- PCR :
-
Polymerase chain reaction
- PNRSV :
-
Prunus necrotic ringspot virus
- PPV :
-
Plum pox virus
- RT-PCR :
-
Reverse transcriptase-PCR
- PVP-40 :
-
Polyvinylpyrrolidone-40
- WPM :
-
Woody plant medium
References
Adams AN (1978) The detection of plum pox virus in Prunus spp. species by enzyme-linked immunosorbent assay (ELISA). Ann Appl Biol 90:215–221
Adams AN, Guise CM, Crossley SJ (1999) Plum pox virus detection in dormant plum trees by PCR and ELISA. Plant Pathol 48:240–244
Ayabe M, Sumi S (2001) A novel and efficient tissue culture method—"stem-disc dome culture"—for producing virus-free garlic (Allium sativum L.). Plant Cell Rep 20:503–507
Brown GR, Kwiatkowski S, Martin MW, Thomas PE (1988) Eradication of PVS from potato clones through excision of meristems from in vitro heat-treated shoot tips. Am Potato J 65:633–638
Candresse T, Macquaire G, Lanne M, Bousalem M, Quiot-Douine L, Quiot JB, Dunez J (1995) Analysis of plum pox virus variability and development of strain-specific assays. Acta Hortic 386:357–369
Crosslin JM, Hammond R, Hammerschlag FA (1992) Detection of prunus necrotic ringspot virus serotypes in herbaceous and Prunus hosts with a complementary RNA probe. Plant Dis 76:1132–1136
Dovas IC, Hatziloucas E, Salomon R, Barg E, Shiboleth Y, Katis NI (2001) Comparison of methods for virus detection in Allium spp. J Phytopathol 149:731–737
Escalettes V, Dosba F, Lansac M, Eyquard JP (1988) Genetic resistance to plum pox potyvirus in peaches. Acta Hortic 465:689–698
Greber RS, Klose MJ, Milne JR, Teakle DS (1991) Transmission of prunus necrotic ringspot using plum pollen and thrips. Ann Appl Biol 118:580–593
Hammerschlag FA, Scorza R (1991) Field performance of micropropagated own-rooted peach trees. J Am Soc Hortic Sci 116:1089–1091
Hammerschlag FA, Bauchan GR, Scorza R (1987) Factors influencing in vitro multiplication and rooting of peach cultivars. Plant Cell Tissue Organ Cult 8:235–242
Helguera PR, Taborda R, Docampo DM, Ducasse DA (2001) Immunocapture reverse transcription-polymerase chain reaction combined with nested PCR greatly increases the detection of prunus necrotic ring spot virus in the peach. J Virol Methods 95:93–100
Heuss K, Liu Q, Hammerschlag FA, Hammond RW (1999) A cRNA probe detects prunus necrotic ringspot virus in three peach cultivars after micrografting and in peach shoots following long-term culture at 4°C. HortScience 34:346–347
Heuss-La Rosa K, Hammond R, Crosslin JM, Hazel C, Hammerschlag FA (1995) Monitoring prunus necrotic ringspot virus infection by hybridization with a cRNA probe after in vitro micrografting. J Am Soc Hortic Sci 120:928–931
Herrera G, Sepulvida P, Madariaga M (1998) Survey of sharka disease (plum pox virus) on stone fruit trees in Chile. Acta Hortic 472:393–399
Kartha KK, Gamborg OL (1975) Elimination of cassava mosaic disease by meristem culture. Phytopathology 65:826–828
Lloyd G, McCown B (1981) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb Proc Intl Plant Prop Soc 30:421–427
Navarro L (1988) Application of shoot-tip grafting in vitro to woody species. Acta Hortic 227:43–55
Pusey PL, Yadava UL (1991) Influence of prunus necrotic ringspot virus on growth, productivity, and longevity of peach trees. Plant Dis 75:847–851
Rosner A, Maslenin L, Spiegel S (1998) Differentiation among isolates of prunus necrotic ringspot virus by transcript conformation polymorphism. J Virol Methods 74:109–115
Rowhani A, Maningas MA, Lile LS, Daubert SD, Golino DA (1995) Development of a detection system for viruses of woody plants based on PCR analysis of immobilized virions. Phytopathology 85:347–352
Scott SW, Barnett OW, Burrows PM (1989) Incidence of prunus necrotic ringspot virus in selected peach orchards of South California. Plant Dis 73:913–916
Shu W, Timon B (1993) Preliminary study on the methods of getting virus-free peach plantlets in vitro. Acta Hortic 374:191–194
Skirvin RM, Kouider M, Joung A, Korban SS (1986) The tissue culture of apple Malus domestica Borkh. In: Bajaj YPS (ed) Trees. Biotechnology in agriculture and forestry, vol 1. Springer, Berlin Heidelberg New York, pp 183–197
Spiegel S, Scott SW, Bowman-Vance V, Tam Y, Galiak-Parov NN, Rosner A (1996) Improved detection of prunus necrotic ringspot virus by the polymerase chain reaction. Eur J Plant Pathol 102:681–685
Uyemoto JK, Asai WK, Luhn F (1992) Ilarviruses: evidence for rapid spread and effects on vegetative growth and fruit yields of peach trees. Plant Dis 76:71–74
Varveri C, Bem F (1995) Viruses of stone and pome fruit mother-tree plantations in Greece. Acta Hortic 386:431–438
Varveri C, Candresse T, Cugusi M, Ravelonandro M, Dunez J (1988) Use of 32P labelled transcribed RNA probe for dot hybridization detection of plum pox virus. Phytopathology 78:1280–1283
Walkey DGA (1980) Production of virus-free plants by tissue culture. In: Ingram DS, Helgeson IP (eds) Tissue culture methods for plant pathologists. Blackwell, Oxford, pp 109–117
Wetzel T, Candresse T, Ravelonandro M, Dunez J (1991) A polymerase chain reaction assay adapted to plum pox potyvirus detection. J Virol Methods 33:355–365
Acknowledgements
The authors would like to thank the Pomology Institute of Naoussa (NAGREF) for the supply of plant material and Dr. T. Syros for technical assistance. GAM thanks the Greek Scholarship Foundation for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.C. Phillips
Rights and permissions
About this article
Cite this article
Manganaris, G.A., Economou, A.S., Boubourakas, I.N. et al. Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep 22, 195–200 (2003). https://doi.org/10.1007/s00299-003-0681-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0681-y