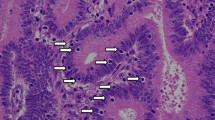
Abstract
The aim of this study was to compare the results of protein level of the DNA mismatch repair genes with the clinical diagnosis of Lynch syndrome according to the Amsterdam II criteria in patients 50 years and younger who underwent surgery for colorectal cancer. The subjects of analysis were 48 patients 50 years old and younger. Immunohistochemistry assays were performed to detect proteins from the DNA mismatch repair genes. Clinicopathological data and Amsterdam II criteria for the diagnosis of hereditary nonpolyposis colorectal cancer were obtained by analyzing medical records. Two (4 %) patients satisfied the Amsterdam II criteria for Lynch syndrome, and both presented levels of all of the studied mismatch repair proteins. A total of 13 (27 %) patients exhibited the absence of protein levels of the studied mismatch repair genes. None of these patients were considered suspicious for Lynch syndrome according to the Amsterdam II criteria. Screening for the level of proteins of the mismatch repair system in all colorectal cancer patients 50 years and younger can increase the identification of patients with suspicion of Lynch syndrome.



Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36.
Martellucci J, Civitelli S, Dhamo A, Tanzini G. Familial colorectal cancer: a concept revisited. Colorectal Dis. 2009;11:133–7.
Abdel-Rahman WM, Peltomäki P. Lynch syndrome and related familial colorectal cancers. Crit Rev Oncog. 2008;14:1–22.
Athanasios T. The clinical molecular diagnostics laboratory and microsatellite instability testing of colorectal cancer. Pathol Case Rev. 2010;15:111–5.
Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–7.
Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009;75:141–9.
Southey MC, Jenkins MA, Mead L, Whitty J, Trivett M, Tesoriero AA, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol. 2005;23:6524–32.
Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, et al. Recommendations to improve identification of hereditary and familial colorectal cancer in Europe. Fam Cancer. 2010;9:109–15.
Talseth-Palmer BA, McPhillips M, Groombridge C, Spigelman A, Scott RJ. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8:5.
Stupart DA, Goldberg PA, Baigrie RJ, Algar U, Ramesar R. Surgery for colonic cancer in HNPCC: total vs segmental colectomy. Colorectal Dis. 2011;13:1395–9.
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18.
Yoon YS, Yu CS, Kim TW, Kim JH, Jang SJ, Cho DH, et al. Mismatch repair status in sporadic colorectal cancer: immunohistochemistry and microsatellite instability analyses. J Gastroenterol Hepatol. 2011;26:1733–9.
Wu Y, Berends MJ, Sijmons RH, Mensink RG, Verlind E, Kooi KA, et al. A role for MLH3 in hereditary nonpolyposis colorectal cancer. Nat Genet. 2001;29:137–8.
Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193–201.
Peltomäki P. Lynch syndrome genes. Fam Cancer. 2005;4:227–32.
Plaschke J, Krüger S, Jeske B, Theissig F, Kreuz FR, Pistorius S, et al. Loss of MSH3 protein expression is frequent in MLH1-deficient colorectal cancer and is associated with disease progression. Cancer Res. 2004;64:864–70.
Hendriks YM, Wagner A, Morreau H, Menko F, Stormorken A, Quehenberger F, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127:17–25.
Huang J, Kuismanen SA, Liu T, Chadwick RB, Johnson CK, Stevens MW, et al. MSH6 and MSH3 are rarely involved in genetic predisposition to nonpolypotic colon cancer. Cancer Res. 2001;61:1619–23.
Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65.
Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419–28.
Rossi BM, Lopes A, Oliveira Ferreira F, Nakagawa WT, Napoli Ferreira CC, Casali Da Rocha JC, et al. hMLH1 and hMSH2 gene mutation in Brazilian families with suspected hereditary nonpolyposis colorectal cancer. Ann Surg Oncol. 2002;9:555–61.
Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, et al. A National Cancer Institute workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–62.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
de Jong AE, van Puijenbroek M, Hendriks Y, Tops C, Wijnen J, Ausems MG, et al. Microsatellite instability, immunohistochemistry, and additional PMS2 staining in suspected hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10:972–80.
Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. J Mol Diagn. 2008;10:293–300.
Edelmann W, Umar A, Yang K, Heyer J, Kucherlapati M, Lia M, et al. The DNA mismatch repair genes Msh3 and Msh6 cooperate in intestinal tumor suppression. Cancer Res. 2000;60:803–7.
Bouzourene H, Hutter P, Losi L, Martin P, Benhattar J. Selection of patients with germline MLH1 mutated Lynch syndrome by determination of MLH1 methylation and BRAF mutation. Fam Cancer. 2010;9:167–72.
Hitchins MP, Ward RL. Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J Med Genet. 2009;46:793–802.
Limburg PJ, Harmsen WS, Chen HH, Gallinger S, Haile RW, Baron JA, et al. Prevalence of alterations in DNA mismatch repair genes in patients with young-onset colorectal cancer. Clin Gastroenterol Hepatol. 2011;9:497–502.
Ganapathi S, Kumar D, Katsoulas N, Melville D, Hodgson S, Finlayson C, et al. Colorectal cancer in the young: trends, characteristics and outcome. Int J Colorectal Dis. 2011;26:927–34.
O’Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;187:343–8.
Koehler-Santos P, Izetti P, Abud J, Pitroski CE, Cossio SL, Camey SA, et al. Identification of patients at-risk for Lynch syndrome in a hospital-based colorectal surgery clinic. World J Gastroenterol. 2011;17:766–73.
Viana DV, Góes JR, Coy CS, Ayrizono MDLS, Lima CS, Lopes-Cendes I. Family history of cancer in Brazil: is it being used? Fam Cancer. 2008;7:229–32.
Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology. 2003;124:544–60.
Boland CR, Shike M. Report from the Jerusalem workshop on Lynch syndrome-hereditary nonpolyposis colorectal cancer. Gastroenterology. 2010;138:2197–e1.
Lindor NM. Familial colorectal cancer type X: the other half of hereditary non polyposis colon cancer syndrome. Surg Oncol Clin N Am. 2009;18:637–45.
Guo J, Zheng L, Liu W, Wang X, Wang Z, Wang Z, et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 2011;71:2978–87.
Geary J, Sasieni P, Houlston R, Izatt L, Eeles R, Payne SJ, et al. Gene-related cancer spectrum in families with hereditary non-polyposis colorectal cancer (HNPCC). Fam Cancer. 2008;7:163–72.
Lynch HT, Albano W, Recerbaren J, Lynch PM, Lynch JF, Elston R. Prolonged survival as a component of a hereditary breast and nonpolyposis colon cancer. Med Hypotheses. 1981;7:1201–9.
Prolla TA, Baker SM, Harris AC, Tsao JL, Yao X, Bronner CE, et al. Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nat Genet. 1998;18:276–9.
Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–8.
Laghi L, Bianchi P, Delconte G, Celesti G, Di Caro G, Pedroni M, et al. MSH3 protein expression and nodal status in MLH1-deficient colorectal cancers. Clin Cancer Res. 2012;18:3142–53.
Takahashi M, Koi M, Balaguer F, Boland CR, Goel A. MSH3 mediates sensitization of colorectal cancer cells to cisplatin, oxaliplatin, and a poly (ADP-ribose) polymerase inhibitor. J Biol Chem. 2011;286:12157–65.
Plaschke J, Preußler M, Ziegler A, Schackert HK. Aberrant protein expression and frequent allelic loss of MSH3 in colorectal cancer with low-level microsatellite instability. Int J Colorectal Dis. 2012;27:911–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Germini, D.E., Mader, A.M.A.A., Gomes, L.G.L. et al. Detection of DNA repair protein in colorectal cancer of patients up to 50 years old can increase the identification of Lynch syndrome?. Tumor Biol. 37, 2757–2764 (2016). https://doi.org/10.1007/s13277-015-4108-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4108-5