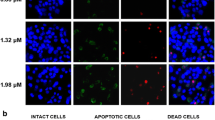
Abstract
Green tea polyphenol (GTP) is one of the most promising chemopreventive agent for cancer; it can inhibit cancer cell proliferation and induce apoptosis through p53-dependent cell signaling pathways. Unfortunately, many tumor cells lack the functional p53, and little is known about the effect of GTP on the p53-deficient/mutant cancer cells. To understand the p53-independent mechanisms in GTP-treated p53-dificient/mutant cancer cells, we have now examined GTP-induced cytotoxicity in human hepatoma Hep3B cells (p53-deficient). The results showed that GTP could induce Bax and Bak activation, cytochrome c release, caspase activation, and necroptosis of Hep3B cells. Bax and Bak, two key molecules of mitochondrial permeability transition pore (MPTP), were interdependently activated by GTP, with translocation and homo-oligomerization on the mitochondria. Bax and Bak induce cytochrome c release. Importantly, cytochrome c release and necroptosis were diminished in Hep3B cells (Bax−/−) and Hep3B cells (Bak−/−). Furthermore, overexpression of Bcl-2 could ameliorate GTP-induced cytochrome c release and necroptosis. Together, the findings suggested that GTP-induced necroptosis was modulated by the p53-independent pathway, which was related to the translocation of Bax and Bak to mitochondria, release of cytochrome c, and activation of caspases.






Similar content being viewed by others
Abbreviations
- GTP:
-
Green tea polyphenol
- DMSO:
-
Dimethyl sulfoxide
- Bax:
-
Bcl-2-associated X protein
- Bak:
-
Bcl-2 antagonist killer
- MPTP:
-
Mitochondrial permeability transition pore
Reference
Thangapazham RL, Passi N, Maheshwari RK. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol Ther. 2007;6:1938–43.
Moseley VR et al. Green tea polyphenol epigallocatechin 3-gallate, contributes to the degradation of DNMT3A and HDAC3 in HCT 116 human colon cancer cells. Anticancer Res. 2013;33:5325–33.
Hofmann CS, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate induces apoptosis of proliferating vascular smooth muscle cells via activation of p53. FASEB J. 2003;17:702–4.
Thakur VS, Gupta K, Gupta S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int J Oncol. 2012;41:353–61.
Gupta K et al. Green tea polyphenols induce p53-dependent and p53-independent apoptosis in prostate cancer cells through two distinct mechanisms. PLoS One. 2012;7:e52572.
Houghton JA. Apoptosis and drug response. Curr Opin Oncol. 1999;11:475–81.
Bai WK, Shen E, Hu B. The induction of the apoptosis of cancer cell by sonodynamic therapy: a review. Chin J Cancer Res. 2012;24:368–73.
Ocker M, Hopfner M. Apoptosis-modulating drugs for improved cancer therapy. Eur Surg Res. 2012;48:111–20.
Tang DG, Porter AT. Apoptosis: a current molecular analysis. Pathol Oncol Res. 1996;2:117–31.
Ghardi M et al. Radiation-induced double strand breaks and subsequent apoptotic DNA fragmentation in human peripheral blood mononuclear cells. Int J Mol Med. 2012;29:769–80.
Peter ME. Programmed cell death: apoptosis meets necrosis. Nature. 2011;471:310–2.
Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43.
McCall K. Genetic control of necrosis— another type of programmed cell death. Curr Opin Cell Biol. 2010;22:882–8.
Lin X et al. Cell-death-mode switch from necrosis to apoptosis in hydrogen peroxide treated macrophages. Sci China Life Sci. 2010;53:1196–203.
Nicotera P, Melino G. Regulation of the apoptosis-necrosis switch. Oncogene. 2004;23:2757–65.
Hong B et al. Targeting tumor suppressor p53 for cancer therapy: strategies, challenges and opportunities. Curr Drug Targets. 2014;15:80–9.
Kosmider B et al. Enhanced P53 and BAX gene expression and apoptosis in A549 cells by cis-Pt(II) complex of 3-aminoflavone in comparison with cis-DDP. Investig New Drugs. 2005;23:287–97.
Lee WT, Chang CW. Bax is upregulated by p53 signal pathway in the SPE B-induced apoptosis. Mol Cell Biochem. 2010;343:271–9.
Manna S et al. Tea polyphenols can restrict benzo[a]pyrene-induced lung carcinogenesis by altered expression of p53-associated genes and H-ras, c-myc and cyclin D1. J Nutr Biochem. 2009;20:337–49.
Hastak K et al. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–91.
Han DH, Jeong JH, Kim JH. Anti-proliferative and apoptosis induction activity of green tea polyphenols on human promyelocytic leukemia HL-60 cells. Anticancer Res. 2009;29:1417–21.
Guo BC, Xu YH. Bcl-2 over-expression and activation of protein kinase C suppress the trail-induced apoptosis in Jurkat T cells. Cell Res. 2001;11:101–6.
Khan N, Mukhtar H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochem Pharmacol. 2013;85:667–72.
Hsu S et al. Green tea polyphenol targets the mitochondria in tumor cells inducing caspase 3-dependent apoptosis. Anticancer Res. 2003;23:1533–9.
Lin J, Della-Fera MA, Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes Res. 2005;13:982–90.
Darzynkiewicz Z et al. DNA damage signaling assessed in individual cells in relation to the cell cycle phase and induction of apoptosis. Crit Rev Clin Lab Sci. 2012;49:199–217.
Co NN et al. AF1q enhancement of gamma irradiation-induced apoptosis by up-regulation of BAD expression via NF-kappaB in human squamous carcinoma A431 cells. Oncol Rep. 2010;24:547–54.
Yamamoto M et al. The p53 tumor suppressor gene in anticancer agent-induced apoptosis and chemosensitivity of human gastrointestinal cancer cell lines. Cancer Chemother Pharmacol. 1999;43:43–9.
Wojciechowski J, Lovborg H, Wesierska-Gadek J. Activation of p53 protein in normal and in tumor cells by a novel anticancer agent CHS 828. Drugs Exp Clin Res. 2003;29:53–67.
Iyer NG et al. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc Natl Acad Sci U S A. 2004;101:7386–91.
Leibowitz BJ et al. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol Cancer Res. 2011;9:616–25.
Gallenne T et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–90.
Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–9.
Holcakova J et al. The cell type-specific effect of TAp73 isoforms on the cell cycle and apoptosis. Cell Mol Biol Lett. 2008;13:404–20.
Satoh S, Arai K, Watanabe S. Identification of a novel splicing form of zebrafish p73 having a strong transcriptional activity. Biochem Biophys Res Commun. 2004;325:835–42.
Bai L et al. ZBP-89-induced apoptosis is p53-independent and requires JNK. Cell Death Differ. 2004;11:663–73.
Prasad S et al. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem. 2011;286:5546–57.
Sundararajan R et al. Tumor necrosis factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol Chem. 2001;276:45120–7.
Mikhailov V et al. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J Biol Chem. 2003;278:5367–76.
Howell KA et al. Oxygen initiation of respiration and mitochondrial biogenesis in rice. J Biol Chem. 2007;282:15619–31.
Nakabayashi J, Sasaki A. A mathematical model for apoptosome assembly: the optimal cytochrome c/Apaf-1 ratio. J Theor Biol. 2006;242:280–7.
Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–7.
Iwasaki R et al. Catechin, green tea component, causes caspase-independent necrosis-like cell death in chronic myelogenous leukemia. Cancer Sci. 2009;100:349–56.
Okada M et al. A novel mechanism for imatinib mesylate-induced cell death of BCR-ABL-positive human leukemic cells: caspase-independent, necrosis-like programmed cell death mediated by serine protease activity. Blood. 2004;103:2299–307.
Baritaud M et al. Histone H2AX: the missing link in AIF-mediated caspase-independent programmed necrosis. Cell Cycle. 2010;9:3166–73.
Acknowledgments
This work was supported by grants from Science Technology Study of Hubei Department of Education Foundation (No. Q20132804). Chinese National Natural Science Foundation (81102363); and the Scientific Research Foundation for the Excellent Middle-Aged and Youth Scientists of Shandong Province of China (BS2012YY037).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, W., Tongyi, S. Role of Bax/Bcl-2 family members in green tea polyphenol induced necroptosis of p53-deficient Hep3B cells. Tumor Biol. 35, 8065–8075 (2014). https://doi.org/10.1007/s13277-014-2064-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2064-0