Abstract
We identify integrin αvβ3 and Fas as receptors for the streptococcal pyrogenic exotoxin B (SPE B), and G308S (SPE B mutant, glycine at residue 308 is changed to serine), which interacts with Fas only, in our previous study. Here, we explore the signal pathways that regulate proapoptotic protein expression after SPE B stimulation. We find that both SPE B and G308S can stimulate the serine phosphorylation of p53, and p53 phosphorylation is inhibited by the anti-Fas antibody but not by anti-αVβ3 antibody. p38 inhibitor and siRNA decrease the activation and translocation of p53 into the nucleus, which executes its transcription activity. These results indicate that after SPE B treatment, p53 is activated and p38 is the upstream of p53. p38 siRNA also decreases the binding of p53 to the bax promoter and interferes with the association of p53 and STAT1. p53, p38, and STAT1 siRNAs downregulate SPE B-induced Bax expression. This shows that SPE B activates the bax promoter via p38/p53 signal pathways through the Fas receptor, and that STAT1 acts as a coactivator of p53. In addition, p38 and p53 siRNAs inhibit SPE B-induced apoptosis. This is consistent with the findings that SPE B upregulates Bax expression through p38/p53 signal pathways that enhance cell apoptosis.





Similar content being viewed by others
References
Musser JM, Hauser AR, Kim MH, Schlievert PM, Nelson K, Selander RK (1991) Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA 88:2668–2672
Schlievert PM, Assimacopoulos AP, Cleary PP (1996) Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J Lab Clin Med 127:13–22
Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, Kaplan E (1989) Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 321:1–7
Chaussee MS, Gerlach D, Yu CE, Ferretti JJ (1993) Inactivation of the streptococcal erythrogenic toxin B gene (speB) in Streptococcus pyogenes. Infect Immun 61:3719–3723
Chen CY, Luo SC, Kuo CF, Lin YS, Wu JJ, Lin MT, Liu CC, Jeng WY, Chuang WJ (2003) Maturation processing and characterization of streptopain. J Biol Chem 278:17336–17343
Hauser AR, Schlievert PM (1990) Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol 172:4536–4542
Kuo CF, Wu JJ, Lin KY, Tsai PJ, Lee SC, Jin YT, Lei HY, Lin YS (1998) Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun 66:3931–3935
Tsai WH, Chang CW, Chuang WJ, Lin YS, Wu JJ, Liu CC, Chang WT, Lin MT (2004) Streptococcal pyrogenic exotoxin B-induced apoptosis in A549 cells is mediated by a receptor- and mitochondrion-dependent pathway. Infect Immun 72:7055–7062
Oren M (2003) Decision making by p53: life, death and cancer. Cell Death Differ 10:431–442
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–310
Miyashita T, Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–694
Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058
Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS (2002) BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol 4:842–849
Wu GS, Burns TF, McDonald ER III, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, El-Deiry WS (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 17:141–143
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7:673–682
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805–816
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D (1993) p21 is a universal inhibitor of cyclin kinases. Nature 366:701–704
Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ Jr (1999) Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18:6845–6854
Thornborrow EC, Manfredi JJ (2001) The tumor suppressor protein p53 requires a cofactor to activate transcriptionally the human BAX promoter. J Biol Chem 276:15598–15608
Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC, Tseng TH (2003) Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol 66:2281–2289
Li DW, Fass U, Huizar I, Spector A (1998) Okadaic acid-induced lens epithelial cell apoptosis requires inhibition of phosphatase-1 and is associated with induction of gene expression including p53 and bax. Eur J Biochem 257:351–361
Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A (2004) STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J Biol Chem 279:5811–5820
Holmstrom TH, Tran SE, Johnson VL, Ahn NG, Chow SC, Eriksson JE (1999) Inhibition of mitogen-activated kinase signaling sensitizes HeLa cells to Fas receptor-mediated apoptosis. Mol Cell Biol 19:5991–6002
Deng Y, Ren X, Yang L, Lin Y, Wu X (2003) A JNK-dependent pathway is required for TNFα-induced apoptosis. Cell 115:61–70
Porras A, Zuluaga S, Black E, Valladares A, Alvarez AM, Ambrosino C, Benito M, Nebreda AR (2004) p38α mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol Biol Cell 15:922–933
Tsai WH, Chang CW, Lin YS, Chuang WJ, Wu JJ, Liu CC, Tsai PJ, Lin MT (2008) Streptococcal pyrogenic exotoxin B-induced apoptosis in A549 cells is mediated through αvβ3 integrin and Fas. Infect Immun 76:1349–1357
Aiyar A, Xiang Y, Leis J (1996) Site-directed mutagenesis using overlap extension PCR. Methods Mol Biol 57:177–191
Chang CW, Tsai WH, Chuang WJ, Lin YS, Wu JJ, Liu CC, Chang WT, Lin MT (2009) Procaspase 8 and Bax are upregulated by distinct pathways in the SPE B-induced apoptosis. J Biol Chem 284:33195–33205
Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT (2005) Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology 129:1875–1888
Wang H, Yadav JS (2007) Global gene expression changes underlying Stachybotrys chartarum toxin-induced apoptosis in murine alveolar macrophages: evidence of multiple signal transduction pathways. Apoptosis 12:535–548
Ossina NK, Cannas A, Powers VC, Fitzpatrick PA, Knight JD, Gilbert JR, Shekhtman EM, Tomei LD, Umansky SR, Kiefer MC (1997) Interferon-γ modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem 272:16351–16357
Dai C, Krantz SB (1999) Interferon γ induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood 93:3309–3316
Moll UM, Wolff S, Speidel D, Deppert W (2005) Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol 17:631–636
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM (2003) p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11:577–590
Schuler M, Green DR (2001) Mechanisms of p53-dependent apoptosis. Biochem Soc Trans 29:684–688
Marchenko ND, Zaika A, Moll UM (2000) Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem 275:16202–16212
Akhtar RS, Geng Y, Klocke BJ, Roth KA (2006) Neural precursor cells possess multiple p53-dependent apoptotic pathways. Cell Death Differ 13:1727–1739
Navas TA, Nguyen AN, Hideshima T, Reddy M, Ma JY, Haghnazari E, Henson M, Stebbins EG, Kerr I, O’Young G, Kapoun AM, Chakravarty S, Mavunkel B, Perumattam J, Luedtke G, Dugar S, Medicherla S, Protter AA, Schreiner GF, Anderson KC, Higgins LS (2006) Inhibition of p38α MAPK enhances proteasome inhibitor-induced apoptosis of myeloma cells by modulating Hsp27, Bcl-XL, Mcl-1 and p53 levels in vitro and inhibits tumor growth in vivo. Leukemia 20:1017–1027
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331
Perfettini JL, Castedo M, Nardacci R, Ciccosanti F, Boya P, Roumier T, Larochette N, Piacentini M, Kroemer G (2005) Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J Exp Med 201:279–289
Sabatini N, Di Giacomo V, Rapino M, Rana R, Garaci G, Cataldi A (2005) JNK/p53 mediated cell death response in K562 exposed to etoposide-ionizing radiation combined treatment. J Cell Biochem 95:611–619
Chang CW, Wu SY, Chuang WJ, Lin YS, Wu JJ, Liu CC, Chang WT, Lin MT (2009) The IL-8 production by streptococcal pyrogenic exotoxin B. Exp Biol Med 234:1316–1326
Acknowledgment
We thank Jonathan Courtenay for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
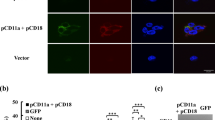
. The level of Bax is not upregulated in p53 null H1299 cell. H1299 cell lysates are extracted and probed with an anti-p53 antibody. The membrane is then reprobed with an anti-β-actin antibody (a). After transfection with p53 reporter, cells (2 × 105) are treated with or without SPE B (20 μg/ml) and the luciferase activity is measured. Data are the means ± SEM of triplicate cultures (b). Cells (3 × 106) are treated with or without SPE B (20 μg/ml) for 5 h. Total RNA is prepared from cells and subjected to RT-PCR analysis of Bax mRNA expression. The cDNA is amplified with specific oligos for Bax and β-actin (c). Cells (2 × 105) are treated with or without SPE B (20 μg/ml) for 10 h. Total cell lysates are probed with an anti-Bax antibody. The membrane is then reprobed with an anti-β-actin antibody (d)
Supplement 2
. Bax translocation into mitochondria is not regulated by p53, and no p53 translocation into mitochondria is detected after SPE B treatment. Cells (2 × 105) are incubated with SPE B (20 μg/ml) with or without siRNA for various times. The mitochondria and cytosol extracts are probed with an anti-Bax antibody (a) or anti-p53 antibody (b). The membrane is then reprobed with an anti-β-actin antibody
Supplement 3
. The mRNA and protein levels of p53 are not increased after SPE B stimulation. Cells (3 × 106) are treated with SPE B (20 μg/ml) for various times. Total RNA is prepared from A549 cells and subjected to RT-PCR analysis of mRNA expression. The cDNA is amplified with specific oligos for p53 and β-actin (a). Cells (2 × 105) are treated with SPE B (20 μg/ml) for various times. Total cell lysates are probed with an anti-p53 antibody. The membrane is then reprobed with an anti-β-actin antibody (b)
Supplement 4
. The mRNA and protein levels of PUMA are not upregulated in the presence of SPE B. Cells (3 × 106) are treated with SPE B (20 μg/ml) for various times. Total RNA is prepared from A549 cells and subjected to RT-PCR analysis of mRNA expression. The cDNA is amplified with specific oligos for PUMA and β-actin (a). Cells (2 × 105) are treated with SPE B (20 μg/ml) for various times. Total cell lysates are probed with an anti-PUMA antibody (b). The membrane is then reprobed with an anti-β-actin antibody
Rights and permissions
About this article
Cite this article
Lee, WT., Chang, CW. Bax is upregulated by p53 signal pathway in the SPE B-induced apoptosis. Mol Cell Biochem 343, 271–279 (2010). https://doi.org/10.1007/s11010-010-0522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0522-6