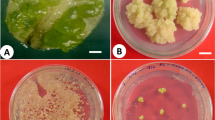
Abstract
Cenchrus ciliaris, commonly known as buffelgrass, is an apomictic perennial range grass usually grown in arid/semi-arid regions. Because of the difficulties faced in conventional breeding of this polymorphic polyploid grass, the development of an efficient protocol for genetic transformation is warranted. Such a protocol would enable functional genomic studies needed to elucidate the mechanism of apomixis in this species. The embryogenic calli for genetic transformation experiments were obtained by in vitro culture of the immature inflorescences of buffelgrass cv. IGFRI-3108. Four developmental stages of immature inflorescences were compared for callus induction, and the most mature stage produced most callus. Embryogenic calli were bombarded at three distances, 6 cm (L1), 9 cm (L2), or 12 cm (L3) with a marker gene uid A present in pCAMBIA1301, under the same vacuum (85 kPa) and at constant pressure (900 psi). The Agrobacterium-mediated genetic transformation was also performed using the same construct. Transient and stable expression as well as PCR amplification of the GUS gene was used for comparative analysis as well as for validation of the transformants. Transient GUS expression was present in a significantly higher percentage of bombarded calli (56.33%) than of Agrobacterium treated calli (11.17%), but the number of GUS positive cells per callus was similar. Among the three different bombardment distances, transient GUS expression was highest at L2, but stable GUS expression was highest at L1. Shoot development from the bombarded calli could be accomplished, which failed from the Agrobacterium-mediated transformed calli. Thus, the results indicate that C. ciliaris cv. IGFRI-3108 can be successfully transformed through Biolistic particle bombardment, while Agrobacterium-mediated transformation requires further optimization of transformation protocols.









Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxy acetic acid
- BAP:
-
6-Benzylaminopurine
- GUS:
-
β-Glucuronidase
- psi:
-
Pound per square inch
- kPa:
-
Kilo pascal
References
Ayerza R. Buffelgrass: utilisation and productivity of a promising grass. Buenos Aires: Hemisferio Sur; 1981.
Batra S, Kumar S. In vitro high frequency plant regeneration in buffelgrass (Cenchrus ciliaris L.). J Plant Biol. 2002;29:191–4.
Batra S, Kumar S. Agrobacterium-mediated transient GUS gene expression in buffel grass (Cenchrus ciliaris L.). J Appl Genet. 2003;44(4):449–58.
Bhat V, Dalton SJ, Kumar S, Bhat BV, Gupta MG, Morris P. Particle-inflow gun-mediated genetic transformation of buffel grass (Cenchrus ciliaris L.): optimizing biological and physical parameters. J Appl Genet. 2001;42:405–12.
Bregitzer P, Campbell RD, Wu Y. Plant regeneration from barley callus: effects of 2,4-dichlorophenoxyacetic acid and phenylacetic acid. Plant Cell Tiss Org. 1995;43:229–35. https://doi.org/10.1007/BF00039949.
Burson BL, Hussey MA, Actkinson JM, Shafer GS. Effect of pollination time on the frequency of 2n+n fertilization in apomictic buffelgrass. Crop Sci. 2002;42(4):1075–80. https://doi.org/10.2135/cropsci2002.1075.
Christou P. Particle bombardment for genetic engineering of plants. Biotechnology Intelligence Unit. UK: AcademicPress; 1996.
Chuanjun X, Zhiwei R, Ling L, Biyu Z, Junmei H, Wen H, Ou H. The effects of polyphenol oxidase and cycloheximide on the early stage of browning in Phalaenopsis explants. Hortic Plant J. 2015;1(3):172–80. https://doi.org/10.16420/j.issn.2095-9885.2015-0030.
El-Dengawy ERFA, Elyazid DMA, Attia AAM. Studies on In vitro surface sterilization and control of phenolics on guava (Psidium guajava L.) nodal explants. Pak J Biotechnol. 2017;43(1):105–14.
Finner JJ, Vain P, Jones MW, Mcmullen MD. Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep. 1992;11:323–8. https://doi.org/10.1007/BF00233358.
Fisher WD, Bashaw EC, Holt EC. Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus. Agron J. 1954;46:401–4.
Green CE, Phillips RL. Plant regeneration from tissue cultures of maize. Crop Sci. 1975;15:417–21.
Ishida Y, Hiei Y, Komari T (2007) Agrobacterium-mediated transformation of maize. Nat Protoc 2(7):1614–1621. https://doi.org/10.1038/nprot.2007.241
Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. https://doi.org/10.2135/cropsci1975.0011183X001500030040x.
Jha P, Yadav CB, Anjaiah V, Bhat V. In vitro plant regeneration through somatic embryogenesis and direct shoot organogenesis in Pennisetum glaucum (L.) R. Br. In Vitro Cell Dev Biol Plant. 2009;45:145–54. https://doi.org/10.1007/s11627-009-9198-6.
Kackar A, Shekhawat NS. Plant regeneration from cultured immature inflorescences of Cenchrus setigerus and C ciliaris. Indian J Exp Biol. 1991;29:62–4.
Kumar J, Shukla SM, Bhat V, Gupta S, Gupta MG. In vitro plant regeneration and genetic transformation of Dichantium annulatum. DNA Cell Biol. 2005;24:670–9. https://doi.org/10.1089/dna.2005.24.670.
Kumar S. Epigenetic control of apomixis: A new perspective of an old enigma. Adv Plants Agric Res. 2017;7(1):227–33. https://doi.org/10.15406/apar.2017.07.00243.
Kumar S, Chandra A. In vitro plantlet regeneration in Stylosanthes spp via callus induction from cotyledonary and hypocotyl explants. Natl Acad Sci Lett. 2010;33:289–97. https://doi.org/10.1016/S2221-1691(14)60206-9.
Kumar S, Arul L, Talwar D, Raina SK. PCR amplification of minimal gene expression cassette: an alternative, low cost and easier approach to clean DNA transformation. Curr Sci. 2006;91:930–4.
Kumar S, Bhat V, Bhat BV, Gupta MG. Agrobacterium-mediated Transformation of lucerne (Medicago sativa Linn): optimizing biological and physical parameters. Indian J Biotechnol. 2002;1:298–300.
Kumar S, Bhat V. High frequency direct plant regeneration via multiple shoot induction in the apomictic forage grass Cenchrusciliaris L. In Vitro Cell Dev Biol Plant. 2012;48:241–8. https://doi.org/10.1007/s11627-012-9428-1.
Kumar S, Sahu N, Singh A. High-frequency in vitro plant regeneration via callus induction in a rare sexual plant of Cenchrusciliaris L. Vitro Cell Dev Biol Plant. 2015;51:28–34. https://doi.org/10.1007/s11627-015-9664-2.
Kumar S, Saxena S. Sequence characterized amplified regions linked with apomictic mode of reproduction in four different apomictic Cenchrus species. Mol Plant Breed. 2016;7:1–14. https://doi.org/10.5376/mpb.2016.07.0008.
Kumar S, Saxena S, Rai A, Radhakrishna A, Kaushal P. Ecological, genetic, and reproductive features of Cenchrus species indicate evolutionary superiority of apomixis under environmental stresses. Ecol Indic. 2019;105:126–36. https://doi.org/10.1016/j.ecolind.2019.05.036.
Martin MH, Cox JR, Ibarra-F F. Climatic effects on buffelgrass productivity in the Sonoram Desert. J Range Manage. 1995;48:60–3.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x.
Nishimura A, Aichi I, Matsuoka M. A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc. 2006;1(6):2796–802. https://doi.org/10.1038/nprot.2006.469.
Rao AS, Singh K, Wight JR. Productivity of Cenchrus ciliarisin relation to rainfall and fertilization. J Range Manage. 1996;49:143–6.
Saha P, Blumwald E (2016) Spike-dip transformation of Setaria viridis. Plant J 86(1):89–101. https://doi.org/10.1111/tpj.13148
Sailaja M, Tarakeswari M, Sujatha M. Stable genetic transformation of castor (Ricinus communis L) via particle gun-mediated gene transfer using embryo axes from mature seeds. Plant Cell Rep. 2008;27:1509–19. https://doi.org/10.1007/s00299-004-0898-4.
Shashi. Developmental morphogenesis and in vitro genetic manipulation of Cenchrus ciliaris L. Thesis. University of Delhi. 2013. https://hdl.handle.net/10603/26730
Sharma KK, Bhatnagar-Mathur P, Thorpe TA. Genetic transformation technology: status and problems. In Vitro Cell Dev Biol plant. 2005;41:102–12. https://doi.org/10.1079/IVP2004618.
Visser NC, Spies JJ, Venter HJT. Apomictic embryo sac development in Cenchrus ciliaris (Panicoideae). Bothalia. 2000;28:83–90.
Yadav CB, Jha P, Mahalakshmi C, Anjaiah V, Bhat V. Somatic embryogenesis and regeneration of Cenchrus ciliaris genotypes from immature inflorescences explants. Biol Plantarum. 2009;53(4):603–9. https://doi.org/10.1007/s10535-009-0111-2.
Ziauddin A, Kasha KJ. Long-term callus cultures of diploid barley (Hordeum vulgare). II. Effect of auxins on chromosomal status of cultures and regeneration of plants. Euphytica. 1990;48:279–86. https://doi.org/10.1007/BF00023662.
Acknowledgements
Laishram Sundari Devi would like to thank University Grants Commission (UGC), India for providing financial assistance in the form of J.R.F and S.R.F. The authors express sincere gratitude to the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), Mumbai (37(1)/14/10/2016-BRNS/37024) for sponsoring this research work by providing financial assistance. We are also grateful to the University of Delhi for Research and Development grant at the initial stage of research work. We would like to thank Director, National Institute of Genome Research (NIPGR), New Delhi and Prof. Rajesh Tandon, Department of Botany, University of Delhi, for providing access to the CIF and laboratory facilities respectively, to perform particle bombardment experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Manoj Prasad.
Rights and permissions
About this article
Cite this article
Laishram, S.D., Goyal, S., Shashi et al. Assessment of biolistic and Agrobacterium-mediated genetic transformation methods in Cenchrus ciliaris. Nucleus 63, 303–312 (2020). https://doi.org/10.1007/s13237-020-00332-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-020-00332-1