Abstract
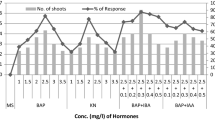
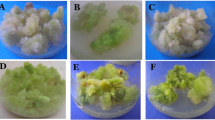
Callus cultures were initiated from shoot base explants of turmeric (Curcuma longa L.) on Murashige and skoog (MS) media supplemented with 2 mg/l 2, 4-dichlorophenoxy acetic acid. Plantlets were regenerated from 90 days old calli on MS media supplemented with 3 mg/l benzyl adenine. Regenerated plants were subsequently transferred to the field. 24 regenerants were analyzed for possible occurrence of somaclonal variation through cytophotometric estimation of 4C nuclear DNA content, analysis of essential oil content and molecular characterization. Significant variation was observed in 4C nuclear DNA content with mean value 8.82 pg and 8.33 pg in 2 regenerants. Analysis of essential oil of 3 regenerants showed significant variation with mean value (0.93 %, 0.73 %, 0.8 %) respectively when compared with the mean oil content 0.48 % of source plant. Inter simple sequence repeat (ISSR) based DNA fingerprinting could also detect variation in regenerants with polymorphism in banding pattern as compared to control. These somaclonal variations detected in callus derived regenerants have enough significance for producing improved varieties of turmeric.


Similar content being viewed by others
References
Bhattacharya S, Dey T, Bandopadhyay TK, Ghosh PD. Genetic polymorphism analysis of somatic embryo derived plantlets of Cymbopogon flexuosus through RAPD assay. Plant Biotechnol Rep. 2008;245–252.
Dey T, Bhattacharya S, Ghosh PD. Somatic embryogenesis from rhizome explants of Cymbopogon winterianus. Biol Plant. 2010;54:325–8.
Flavell RB, Rimpau J, Smith DB. Repeated sequence DNA relationships in four cereal genomes. Chromosoma. 1977;63:5–22.
Godwin ID, Sangduen N, Kunanuvatchaidach R, Piperidis G, Adkins SW. RAPD polymorphisms among variant and phenotypically normal rice (Oryza sativa var.indica) somaclonal progenies. Plant Cell Rep. 1997;16:320–4.
Gupta SP. Statistical methods. New Delhi: Sultan Chand and Sons Publishers; 1987.
Kar DK, Sen S. Effect of hormone on chromosome behaviour in callus cultures of Asparagus racemosus. Biol Plant. 1985;27:6–9.
Kuanar A, Mohanty S, Panda MK, Nayak S. Essential oils from leaves of micro propagated turmeric. Curr Sci. 2009;96:1166–7.
Larkin PJ, Scowcroft WR. Somaclonal variation? A novel source of variability from cell cultures for plant improvement. Theor Appl Genet. 1981;60:197–214.
Mathur AK, Ahuja PS, Pandey B, Kukreja AK, Mandal S. Screening and evaluation of somaclonal variation for quantitative and qualitative traits in aromatic grass, Cymbopogon winterianus Jowitt. Plant Breed. 1988;101:321–34.
Mohanty S, Panda MK, Subudhi E, Nayak S. Plant regeneration from callus culture of Curcuma aromatica and in vitro detection of somaclonal variation through cytophotometric analysis. Biol Plant. 2008;52:783–6.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–9.
Munthali MT, Newbury HJ, Ford-lloyd BV. The detection of somaclonal variants of beet using RAPD. Plant Cell Rep. 1996;15:474–8.
Nayak S, Sen S. Cytological and cytophotometric analysis of direct explant and callus derived plants of Ornithogalum thyrsoides Jacq. Cytologia. 1991;56:297–302.
Nayak S, Kaur T, Mohanty S, Ghosh G, Choudhury R, Acharya L, Subudhi E. In-vitro and ex-vitro evaluation of long term micro propagated turmeric as analyzed through cytophotometry, phytoconstituents, biochemical and molecular markers. Plant Grow Regul. 2011;64:91–8.
Nayak S, Debata BK, Srivastava VK, Sang wan NS. Evaluation Of agronomically useful somaclonal variants in Jamrosa (a hybrid Cymbopogon) and detection of genetic changes through RAPD. Plant Sci. 2003;164:1029–35.
Nayak S, Sen S. Growth, chromosome number and DNA content of callus tissues as influenced by different auxins. Cytobios. 1993;79:209–16.
Nayak S, Sen S. Cytological and cytophotometrical analysis of callus and regenerated plantlets of Ornthogallum virens. Cytobios. 1997;91:135–42.
Patnaik J, Sahoo S, Debata BK. Somaclonal variation in cell suspension culture derived regenerants of Cymbopogon martini (Roxb.) Wats var.motia. Plant Breed. 1999;118:351–4.
Patnaik J, Debata BK. RAPD analysis of variant somaclones of Palmasosa (Cymbopogon martini var.motia). In: Conservation and utilization of medicinal and aromatic plants. Allied publishers limited; 2001. 295–301.
Peredo EL, Arroyo-Garcia R, Revilla MA. Epigenetic changes detected in micro propagated hop plants. J Plant Physiol. 2009;166(10):1101–11.
Potter R, Jones MGK. An assessment of genetic stability of potato in vitro by molecular and phenotypic analysis. Plant Sci. 1991;76:239–48.
Roses IA. Curcuma longa in medicinal plants of the world, chemical constituents, traditional and modern medicinal uses. Totowa: Humana Press; 1999. p. 139–53.
Saha S, Kader A, Sengupta C, Ghosh PD. In vitro propagation of Ocimum gratissimum L. (Lamiaceae) and its evaluation of genetic fidelity using RAPD marker. Am J Plant Sci. 2012;3:64–74.
Sakamura F, Suga T. Zingiber officinale Rosc (ginger). In: Bajaj YPS, editor. Vitro propagation and the production of volatile constituents, in biotechnology in agriculture and forestry, medicinal and aromatic plants 11. Berlin: Springer-Verlag; 1989. 7:524–538.
Sangwan RS, Sangwan NS, Jain DC, Kumar S, Ranade SA. RAPD profile based genetic characterization of chemotypic variants of Artemisia annua L. Biochem Mol Biol Int. 1999;47:935–94.
Sasikumar B. Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genet Resour Characteriz Util. 2005;3:230–51.
Sharma and Sharma. Chromosome techniques: theory and practice. Third edition. London: Butterworths; 1980. 9–27.
Singh S, Kuanar A, Mohanty S, Subudhi E, Nayak S. Evaluation of phytomedicinal yield potential and molecular profiling of micro propagated and conventionally grown turmeric (Curcuma longa L.). Plant Cell Tissue Organ Cult. 2010;104(2):263–9.
Van’t Hof J. Relationships between mitotic cycle duration, S period duration and the average rate of DNA synthesis in the root meristem cells of several plants. Exp Cell Res. 1965;39:48–58.
Vogel AL. In: Textbook of practical organic chemistry. England: English language Book Society, Longman Group Ltd.; 1986. 143–146.
Acknowledgments
The authors are grateful to Dr. S.C. Si, Dean, Center of Biotechnology and Dr.M.R. Nayak, President, Siksha O Anusandhan University for providing. Financial assistance from Department of Science and Technology, New Delhi, India, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ananya Kuanar and Basudeba Kar have equal contribution.
Rights and permissions
About this article
Cite this article
Kuanar, A., Kar, B., Acharya, L. et al. Nuclear DNA, DNA finger printing and essential oil content variation in callus derived regenerants of Curcuma longa L.. Nucleus 55, 101–106 (2012). https://doi.org/10.1007/s13237-012-0065-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-012-0065-1