Abstract
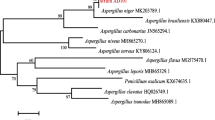
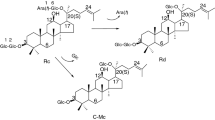
Pathogenic fungus Penicillium oxalicum sp. 68 was screened from soil and identified by ITS sequencing. The strain was found to be able to transform protopanaxadiol-type ginsenosides to produce a series of bioactive metabolites. Glycosidase from the culture of P. oxalicum sp. 68 was partially purified with a simple two-step procedure consisting of DEAE-cellulose chromatography and ammonium sulfate precipitation. Bioactive ginsenoside Compound K was prepared selectively and efficiently by biotransformation of ginsenosides Rb1, Rb2, Rc and Rd using the partially purified glycosidase. The optimal conditions for transforming Rb1 into Compound K were pH 4.0, 55 °C and 0.5 mg mL−1 Rb1. The sole product is Compound K and the maximum yield reached 87.7 % (molar ratio). The transformation pathways of Rb1, Rb2, Rc and Rd are Rb1→Rd→F2→Compound K, Rb2→CO→CY→Compound K, Rc→Mb→Mc→Compound K and Rd→F2→Compound K, respectively. This biotransformation method showed great potential for preparing minor bioactive ginsenosides, especially Compound K, in the pharmaceutical industry because of its high specificity and favorable environmental compatibility.






Similar content being viewed by others
References
Akao T, Kanaoka M, Kobashi K (1998a) Appearance of compound K, a major metabolite of Ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration-measurement of compound K by enzyme immunoassay. Biol Pharm Bull 21:245–249
Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K (1998b) Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol 50:1155–1160
Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH (2002) Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull 25:743–747
Chen Y, Nose M, Ogihara Y (1987) Alkaline cleavage of ginsenosides. Chem Pharm Bull 35:1653–1655
Cheng LQ, Kim MK, Lee JW, Lee YJ, Yang DC (2006) Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol Lett 28:1121–1127
Chi H, Kim DH, Ji GE (2005) Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol Pharm Bull 28:2102–2105
Choi K, Kim M, Ryu J, Choi C (2007) Ginsenosides compound K and Rh2 inhibit tumor necrosis factor-α-induced activation of the NF-κB and JNK pathways in human astroglial cells. Neurosci Lett 421:37–41
Han M, Fang XL (2006) Difference in oral absorption of ginsenoside Rg1 between in vitro and in vivo models. Acta Pharmacol Sin 27:499–505
Han BH, Park MH, Han YN (1982) Degradation of ginseng saponins under mild acidic conditions. Planta Med 44:146–149
Han Y, Sun BS, Hu XM, Zhang H, Jiang BH, Spranger MI, Zhao YQ (2007) Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. J Agric Food Chem 55:9373–9379
Hasegawa H (2004) Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty Acid. J Pharmacol Sci 95:153–157
Ko SR, Suzuki Y, Suzuki K, Choi KJ, Cho BG (2007) Marked production of ginsenosides Rd, F2, Rg3, and Compound K by enzymatic method. Chem Pharm Bull 55:1522–1527
Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH (2005) Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int 25:1069–1073
Lei J, Li X, Gong X, Zheng Y (2007) Isolation, synthesis and structures of cytotoxic ginsenoside. Molecules 12:2140–2150
Lindsley MD, Hurst SF, Iqbal NJ, Morrison CJ (2001) Rapid identification of dimorphic and yeast-like fungal pathogens using specific DNA probes. J Clin Microbiol 39:3505–3511
Muffler K, Leipolda D, Scheller MC, Haas C, Steingroewer J, Bley T, Ekkehard NH, Mirata MA, Schrader J, Ulber R (2011) Biotransformation of triterpenes. Process Biochem 46:1–15
Noh KH, Son JW, Kim HJ, Oh DK (2009) Ginsenoside Compound K production from ginseng root extract by a thermostable β-glycosidase from Sulfolobus solfataricus. Biosci Biotechnol Biochem 73:316–321
Park SY, Bae EA, Sung JH, Lee SK, Kim DH (2001) Purification and characterization of ginsenoside Rb1-metabolizing β-glucosidase from Fusobacterium K-60, a human intestinal anaerobic bacterium. Biosci Biotechnol Biochem 65:1163–1169
Park CS, Yoo MH, Noh KH, Oh DK (2010) Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol 87:9–19
Qian TX, Cai ZW (2010) Biotransformation of ginsenosides Rb1, Rg3 and Rh2 in rat gastrointestinal tracts. Chinese Med 5:19
Ruan CC, Zhang H, Zhang LX, Zhi L, Sun GZ, Lei J, Qin YX, Zheng YN, Li X, Pan HY (2009) Biotransformation of ginsenoside Rf to Rh1 by recombinant β-glucosidase. Molecules 14:2043–2048
Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M (2003) Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos 31:1065–1071
Yan Q, Zhou W, Shi XL, Zhou P, Ju DW, Feng MQ (2010) Biotransformation pathways of ginsenoside Rb1 to compound K by β-glucosidases in fungus Paecilomyces Bainier sp. 229. Process Biochem 45:1550–1556
Yu HS, Zhang CZ, Lu MC, Sun F, Fu YY, Jin FX (2007) Purification and characterization of new special ginsenosidase hydrolyzing multi-glycisides of protopanaxadiol ginsenosides, ginsenosidase type I. Chem Pharm Bull 55:231–235
Zhao XS, Wang J, Li J, Fu L, Gao J, Du XL, Zhou YF, Tai GH (2009) Highly selective biotransformation of ginsenoside Rb1 to Rd by phytopathogenic fungi Cladosporium fulvum (syn. Fulvia fulva). J Ind Microbiol Biotechnol 36:721–726
Zhou W, Feng MQ, Li JY, Zhou P (2006) Studies on the preparation, crystal Structure and bioactivity of ginsenoside compound K. J Asian Nat Prod Res 8:519–527
Zhou W, Yan Q, Li JY, Zhang XC, Zhou P (2008) Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces Bainier sp. 229. J Appl Microbiol 104:699–706
Zhou W, Feng MQ, Li XW, Yan Q, Zhou C, Li JY, Zhou P (2009) X-Ray structure investigation of (20S)-20-O-β-d-glucopyranosyl-protopanaxadiol and antitumor effect on Lewis lung carcinoma in vivo. Chem Biodivers 6:380–388
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 30770489 and 30973857) and the Natural Science Foundation of Jilin Province (200905106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, J., Xu, W., Fang, Q. et al. Efficient biotransformation for preparation of pharmaceutically active ginsenoside Compound K by Penicillium oxalicum sp. 68. Ann Microbiol 63, 139–149 (2013). https://doi.org/10.1007/s13213-012-0454-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-012-0454-3