Abstract
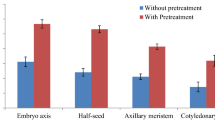
Influence of cytokinins, silver nitrate (AgNO3) and auxins on plant regeneration from cucumber was investigated. The cotyledonary node explants were cultured on MS medium augmented with various concentrations (0.5–2.5 mg l−1) of 6-benzyl amino purine (BAP) and kinetin (KIN) for shoot bud induction. BAP at 1.5 mg l−1 was found to be the best concentration for induction of high frequency of multiple shoots (98.4%). Interestingly, maximum percent of multiple shoot regeneration (100%) as well as number of shoot buds (54.6 shoots/culture) was recorded on MS medium containing the combination of 4.5 mg l−1 AgNO3 and 1.5 mg l−1 BAP. Multiple shoot bud regeneration frequency as well as the number of shoots was positively correlated with the concentrations of AgNO3. Addition of silver nitrate in the medium not only enhanced the rate of multiple shoot bud regeneration but also elongation of shoot buds was observed. The highest percent of rooting (96.2%) was noticed on a medium containing the combination of indole 3-butyric acid (IBA), 1.5 mg l−1 and KIN 0.5 mg l−1. Acclimatized plantlets were successfully established in the field where the survival rate observed was 72%. The RAPD profiles of in vitro regenerated plants were found to be highly monomorphic and identical banding pattern with mother plant. DNA fingerprinting results confirmed that the tissue culture plantlets were found to be true-to-type. The present study describes efficient protocol for high frequency plant regeneration via adventitious shoot organogenesis in cucumber.


Similar content being viewed by others
Abbreviations
- BAP:
-
6-Benzyl amino purine
- KIN:
-
Kinetin
- IBA:
-
Indole 3-butyric acid
- IAA:
-
Indole 3-acetic acid
- NAA:
-
α-Naphthalene acetic acid
- MS:
-
Murashige and Skoog
- AgNO3 :
-
Silver nitrate
- RAPD:
-
Random Amplified Polymorphic DNA
References
Ahmad A, Anis M (2005) In vitro mass propagation of Cucumis sativus L. from nodal segments. Turk J Bot 29:237–240
Al-Zahin MA, Ford-Llyod BV, Newbury HJ (1999) Detection of somaclonal variation in garlic (Allium sativum L.) using RAPD and cytological analysis. Plant Cell Rep 18:473–477
Balkhande SV, Kure SR, Surwase BS (2013) Influence of silver nitrate on shoot regeneration from excised meristems of Momordica cymbalaria Hook.: a diminishing species. Res J Biotech 8:42–47
Baskaran P, Velayutham P, Jayabalan N (2009) In vitro regeneration of Melothria maderaspatana via indirect organogenesis. In Vitro Cell Dev Biol Plant 45:407–413
Compton ME, Pierson BL, Staub JK (2001) Micropropagation for recovery of Cucumis hystrix. Plant Cell Tissue Organ Cult 64:63–67
Devarumath RM, Doule RB, Kawar PG, Naikebawane SB, Nerkar YS (2007) Field performance and RAPD analysis to evaluate genetic fidelity of tissue culture raised plants of sugarcane. Sugar Tech 9:17–22
DeVerno LL, Park YS, Bonga JM, Barrett JD (1999) Somaclonal variation in cryopreserved embryogenic clones of white spruce [Picea glauca (Moench) Voss]. Plant Cell Rep 18:948–953
Diao W, Jia Y, Hio S, Zhang X, Lou Q, Chen JF (2009) Efficient embryo induction in cucumber ovary culture and homozygous identification of the regenerants using SSR markers. Sci Hortic 119:246–251
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Elmeer KMS, Gallagher TF, Hennerty MJ (2009) RAPD-based detection of genomic instability in cucumber plants derived from somatic embryogenesis. Afr J Biotechnol 8:3219–3222
Fatima N, Ahmad N, Anis M (2012) In vitro propagation of Cuphea procumbens Orteg. and evaluation of genetic fidelity in plantlets using RAPD markers. J Plant Biochem Biotechnol 21:51–57
Gambley RL, Dodd WA (1990) An in vitro technique for the production de novo of multiple shoots in cotyledon explants of cucumber (Cucumis sativus L.). Plant Cell Tissue Organ Cult 20:177–183
Ganesan K, Huyop F (2010) In vitro regeneration of Citrullus lanatus cv. Round dragon. J Biol Sci 10:131–137
Giridhar P, Obul Reddy B, Ravishankar GA (2001) Silver nitrate influences in vitro shoot multiplication and root formation in Vanilla planifolia Andr. Curr Sci 81:1166–1170
Giridhar P, Indu E, Vijaya Ramu D, Ravishankar G (2003) Effect of silver nitrate on in vitro shoot growth of coffee. Trop Sci 43:144–146
Grozeva S, Velkov N (2014) In vitro plant regeneration of two cucumber (Cucumis sativum l.) genotypes: effects of explant types and culture medium. Genetika 46:485–493
Han JS, Oh DG, Mok IG, Park HG, Kim CK (2004) Efficient plant regeneration from cotyledon explants of bottle gourd (Lagenaria siceraria Standl.). Plant Cell Rep 23:291–296
Hussain Z, Tyagi RK, Sharma R, Agrawal A (2008) Genetic diversity in in vitro-conserved germplasm of Curcuma L. as revealed by RAPD markers. Biol Plant 52:627–633
Jesmin R, Mian MAK (2016) Callus induction and efficient plant regeneration in Cucumber (Cucumis sativus L.). J Biosci Agri Res 9:796–803
Kawiak A, Lojkowska E (2004) Application of RAPD in the determination of genetic fidelity in micropropagated Drosera plantlets. In Vitro Cell Dev Biol Plant 40:592–595
Khan H, Siddique I, Anis M, Khan PR (2011) In vitro organogenesis from internode derived callus cultures of Capsicum annuum L. J Plant Biochem Biotech 20:84–89
Kontas J, Kintzios S (2003) Developing a scale up system for the micropropagation of cucumber (Cucumis sativus L.). The effect of growth retardants, liquid culture and vessel size. Plant Cell Rep 21:538–548
Kumar V, Parvatam G, Ravishankar GA (2009) AgNO3—a potential regulator of ethylene activity and plant growth modulator. Electron J Biotechnol. https://doi.org/10.2225/vol12-issue2-fulltext-1
Kumar P, Gambhir G, Gaur A, Srivastava DK (2015) Molecular analysis of genetic stability in in vitro regenerated plants of broccoli (Brassica oleracea L. var. italica). Curr Sci 109:1470–1475
Martins M, Sarmento D, Oliveira MM (2004) Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Mashayekhi K, Sharifani M, Shahsavand M, Kalati H (2008) Induction of somatic embryogenesis in absence of exogenous auxin in cucumber. Int J Plant Prod 2:163–166
Mohammadi J, Sivritepe N (2007) In vitro clonal propagation of Cucumis sativus L. by shoot tip culture. J Biol Sci 7:653–657
Mohiuddin AKM, Zaliha C, Abdullah M, Chowdhury KU, Napis S (2005) Enhancement of adventitious shoot regeneration in Cucumis sativus L. using AgNO3. Plant Tissue Cult 15:15–23
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ntui VO, Thirukkumaran G, Lioka S, Mii M (2009) Efficient plant regeneration via organogenesis in Egusi Melon (Colocynthis citrullus L.). Sci Hort 119:397–402
Park EH, Bae H, Park WT, Kim YB, Chae SC, Park SU (2012) Improved shoot organogenesis of gloxinia (Sinningia speciosa) using silver nitrate and putrescine treatment. Plant Omics J 5:6–9
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA 91:5222–5226
Pirinc V, Onay A, Yildirim H, Adiyaman F, Isikalan C, Basaran D (2003) Adventitious shoot organogenesis and plant regeneration from cotyledonasu of diploid diyarbakir watermelon (Citrullus lanatus cv. “Surme”). Turk J Biol 27:101–105
Prem Kumar G, Sivakumar S, Siva G, Vigneswaran M, Senthil Kumar T, Jayabalan N (2016) Silver nitrate promotes high-frequency multiple shoot regeneration in cotton (Gossypium hirsutum L.) by inhibiting ethylene production and phenolic secretion. In Vitro Cell Dev Biol Plant 52:408–418
Qin Y, Li HL, Guo YD (2006) High frequency embryogenesis, regeneration of broccoli (Brassica oleracea var. italica) and analysis of genetic stability by RAPD. Sci Hort 111:203–208
Rathore MS, Mastan SG, Yadav P, Bhatt VD, Shekhawat NS, Chikara J (2016) Shoot regeneration from leaf explants of Withania coagulans (stocks) Dunal and genetic stability evaluation of regenerates with RAPD and ISSR markers. South Afr J Bot 102:12–17
Saini RK, Shetty NP, Giridhar P, Ravishankar GA (2012) Rapid in vitro regeneration method for Moringa oleifera and performance evaluation of field grown nutritionally enriched tissue cultured plants. 3 Biotech 2:187–192
Samantaray S, Maiti S (2010) An assessment of genetic fidelity of micropropagated plants of Chlorophytum borivilianum Santpau and Fernandes using random amplified polymorphic DNA (RAPD) markers. Biol Plant 54:334–338
Selvaraj N, Vasudevan A, Prem Anand R, Ramesh Anbazhgan V, Ganapathi A (2002) Micropropagation of Cucumis sativus L. from field grown plants. In: Proceedings of Cucurbitaceae conference. Naples, pp 149–156
Selvaraj N, Vasudevan A, Manickavasagam V, Ganapathi A (2006) In vitro organogenesis and plant formation in cucumber. Biol Plant 50:123–126
Selvaraj N, Vasudevan A, Manickavasagam M, Kasthurirengan S, Ganapathi A (2007) High frequency shoot regeneration from cotyledon explants of cucumber via organogenesis. Sci Hort 112:2–8
Singh BP, Dubey KK, Singh RP (2007) In vitro direct regeneration of multiple shoots from cotyledon explants of Cucumis sativus L. Plant Arch 7:103–107
Siva G, Sivakumar S, Prem Kumar G, Vigneswaran M, Vinoth S, Arunachalam S, Elango B, Senthil Kumar T, Jayabalan N (2015) Multiple shoot production from nodal explants and FTIR analysis of in vitro regenerated plants of Psoralea corylifolia L. Pharm Biol Eval 2:105–109
Sridevi V, Giridhar P (2014) Establishment of somaclonal variants of Robusta coffee with reduced levels of cafestol and kahweol. In Vitro Cell Dev Biol Plant 50:618–626
Thiyagarajan M, Venkatachalam P (2012) Evaluation of the genetic fidelity of in vitro propagated natural sweetener plant (Stevia rebaudiana Bert.) using DNA-based markers. Plant Cell Biotech Mol Biol 13:99–104
Thomas TD, Sreejesh KR (2004) Callus induction and plant regeneration from cotyledonary explants of ash gourd (Benincasa hispida L.). Sci Hort 100:359–367
Ugandhar T, Venkateshwarrlu M, Begum G, Srilatha T, Jaganmohanreddy K (2011) In vitro plantregeneration of Cucumber (Cucumis sativum L.) from cotyledon and hypocotyl explants. Sci Res Rep 1:164–169
Vasudevan A, Selvaraj N, Sureshkumar P, Ganapathi A (2001) Multiple shoot induction from the shoot tip explants of cucumber (Cucumis sativus L.). Cucurbit Genet Cooperative Rep 24:8–12
Vasudevan A, Selvaraj N, Ganapathi A, Kasthurirengan S, Ramesh Anbazhagan V, Manickavasagam M (2004) Glutamine: a suitable nitrogen source for enhanced shoot multiplication in Cucumis sativus L. Biol Plant 48:1215–1218
Vasudevan A, Selvaraj N, Ganapathi A, Choi CW, Manickavasagam M, Kasthurirengan S (2007) Direct plant regeneration from cucumber embryonal axis. Biol Plant 51:521–524
Vengadesan G, Selvaraj N, Prem Anand R, Gaba V, Ganapathi A (2005) Ontogeny of somatic embryos in Cucumber (Cucumis sativus L.). In Vitro Cell Dev Biol Plant 41:789–793
Venkatachalam P, Sangeetha P, Geetha N, Sahi SV (2015) Phytofabrication of bioactive molecules encapsulated metallic silver nanoparticles from Cucumis sativus L. and its enhanced wound healing potential in rat model. J Nanomater. https://doi.org/10.1155/2015/753193
Walden R, Wingender R (1995) Gene-transfer and plant-an easy-going general review. Regeneration techniques. Trends Biotechnol 13:324–331
Wang SL, Seong SK, Ye XG, He CF, Suk YK, Pil SC (2015) Current status of genetic transformation technology developed in cucumber (Cucumis sativus L.). J Integrat Agric 14:469–482
Werner ET, Soares TCB, Gontijo ABPL, Souza Neto JD, do Amaral JAT (2015) Genetic stability of micropropagated plants of Crambe abyssinica Hochst using ISSR markers. Genet Mol Res 14:16450–16460
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Zhang P, Phansiri S, Puonti-Kaerlas J (2001) Improvement of cassava shoot organogenesis by the use of silver nitrate in vitro. Plant Cell Tiss Org Cult 67:45–54
Acknowledgements
Our sincere thanks to University Grants Commission, Govt. of India, New Delhi for providing UGC-BSR Research fellowship (URF) to P. Sangeetha.
Author information
Authors and Affiliations
Contributions
PV, PS Collected samples, conducted experiments; PV, UJ, PS, NG, SVS Analyzed the data and critically reviewed the manuscript, discussed the results and implications on the manuscript at all stages.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Venkatachalam, P., Jinu, U., Sangeetha, P. et al. High frequency plant regeneration from cotyledonary node explants of Cucumis sativus L. cultivar ‘Green Long’ via adventitious shoot organogenesis and assessment of genetic fidelity by RAPD-PCR technology. 3 Biotech 8, 60 (2018). https://doi.org/10.1007/s13205-018-1083-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1083-8