Abstract
Incorporating zinc oxide nanoparticles (ZnO NPs) into cement mortars may provide additional functions, e.g., self-cleaning and antibacterial or electroconductive ability. However, these NPs are also known for their potential toxicity. During the life cycle of a cement mortar, various abrasive forces cause the release of admixtures to the natural environment. The effect of the released NPs on model microorganisms has not been extensively studied. Previous studies have shown that nanomaterials may affect various microorganisms’ physiological responses, including changes in metabolic activity, biofilming, or growth rate. In this study, we have focused on evaluating the response of model microorganisms, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans, towards ZnO nanoparticles released from cement mortars in different deterioration scenarios. The addition of ZnO nanoparticles to cement mortars had a noticeable effect on impeding the strength development. We have also detected that depending on the deterioration scenario, the release of ZnO nanoparticles was varied. Our studies have also shown that even though the release of nanoform ZnO could be limited by poor dispersion or the used filtration technique, the eluates have caused slight but statistically significant changes in the physiological features of studied microorganisms showing relatively low toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The modification of cement-based composites by including nanoparticles (NPs) has gathered spectacular attention for the last two decades. Despite the significant amount of research works and the first commercially available products on the market, occupational safety, and health-related issues regarding handling and exposure to nanoparticles are still unclear and fully understood. During various internal and external conditions over the life cycle of building structures, cementitious composites undergo a series of deterioration and weathering processes, thus the possibility of releasing of nanomaterials should be carefully studied (Lee et al. 2010; Van Broekhuizen et al. 2011; Jones et al. 2016; Silva et al. 2018; Giese et al. 2018; Augustyniak et al. 2019). Another aspect that was rarely considered is the interaction between various human-made materials and microorganisms, even though they have multiple meanings, e.g., for the sustainability of cementitious materials (Holden et al. 2014; Sikora et al. 2018a).
Various NPs are introduced to cementitious composites, including SiO2, Al2O3, TiO2, carbon nanotubes (CNTs), graphene oxide (GO), Fe3O4, and ZnO. Depending on the type of NP used, various beneficial modifications of cementitious composites can be achieved. For instance, ZnO nanoparticles are used for altering a vast range of composites’ properties, including retardation of hydration and setting time of cement (Senff et al. 2014; Liu et al. 2019; Siler et al. 2019; Osman et al. 2020), modification of rheological parameters (Ghafari et al. 2016), improving long-term mechanical performance (Nazari and Riahi 2011; Thangapandi et al. 2020), increasing impermeability of the cement matrix (Nazari and Riahi 2011; Arefi and Rezaei-Zarchi 2012; Kamal et al. 2020) as well as producing composites with self-cleaning and antibacterial (Senff et al. 2014; Elia and Nima 2019; Le Pivert et al. 2019) or electroconductive ability (Ghahari et al. 2017).
Despite the beneficial effects of ZnO nanoparticles on cementitious composites’ performance, there is a growing concern related to the effects that it can have on living organisms when released (Mielcarz-Skalska and Smolińska 2017; Ganguly et al. 2018). For example, the exposition of E. coli or Salmonella Typhimurium to zinc oxide and titanium dioxide nanoparticles cause osmotic stress that can influence the shape of cytoplasmic membranes (Kumar et al. 2011a; Butler et al. 2014). It was also shown that NPs could influence soil microbiota’s composition when released to the natural environment, where the addition of metal oxide nanoparticles led to changes in soil microbiome (Ge et al. 2011). The disruption of microbiota can influence cause, e.g., interruptions in the cycles of elements (sulfur, phosphorus, nitrogen, and carbon) (Philippot et al. 2013). On the other hand, nanomaterials’ admixtures may be used to change microorganisms’ behavior that should be further studied to use these phenomena in applied sciences (e.g., biotechnology) (Augustyniak et al. 2016, 2020a).
Nanomaterials were found to exhibit certain toxicity when analyzed in the laboratory scale; however, the assessed toxicity of particular NPs cannot be directly transferred from laboratory results to the real release scenarios. Since NPs are immobilized within the cement matrix, there is a high possibility that NPs during the deterioration of composite will be released together with matrix particles or in an agglomerated form (Nowack and Bucheli 2007; Mitrano et al. 2015; Giese et al. 2018; Augustyniak et al. 2019). Moreover, obtaining the full dispersion of NPs when producing cementitious composites is a challenging issue. It is attributed to relatively simple concrete/mortar production process when compared to other fields of sciences, which consists of (i) limited amount of water available for dispersion of NPs (limited by water–cement ratio); (ii) generally high dosage of NPs (usually up to 5% by weight of cement); (iii) simple dispersion techniques (usually limited to ultrasonication); (iv) scale of production which facilitates (support) the agglomeration of the material. Our previous study showed that in the conditions mentioned above, reaching the uniform dispersion of metal oxide NPs used to modify cement-based composites is far from ideal (Sikora et al. 2018a). Thus, the toxic effects of water suspension microorganisms containing NPs (Fe3O4, Al2O3, CuO), prepared towards mixing it with dry components of cementitious composites, were relatively limited. However, these studies indicated that some NPs such as ZnO exhibited possible toxic characteristics and that the reaction to the used metal oxides depended on the used microbial strain (Sikora et al. 2018a).
In recent years, there is a growing concern about the safety of using NPs in cementitious composites. Thus, the amount of the research works increased substantially (Bräu et al. 2012; Spinazzè et al. 2016; Bossa et al. 2017, 2019; Diamond et al. 2017; Miller et al. 2017; Strokova et al. 2018; Funk et al. 2019; Torres-Carrasco et al. 2019; Augustyniak et al. 2020b). In Funk et al. (2019) analyzed micro- and nano-components’ release from cementitious composites containing TiO2 and SiO2 NPs after freeze-thawing. In works (Bossa et al. 2017, 2019; Diamond et al. 2017), extensive evaluations on the release behavior and understanding the phenomena underlying the mechanism on releasing TiO2 nanoparticles were performed. In our recent research (Augustyniak et al. 2020b), the effects of commercially available and laboratory synthesized calcium-silicate-hydrate (C-S-H) nanostructures on the reference microorganisms were tested. These studies have shown that there, in some cases, the addition of C-S-H changes the growth environment for bacteria in a way that promotes their growth (Augustyniak et al. 2020b). However, this effect depended on the type of admixture and a particular bacterial strain. Nevertheless, these results have highlighted a necessity further to study the physiological response of microorganisms to nanomaterials.
Guidelines considering the evaluation of potential toxicity and other physiological effects of NPs used in cement-based materials are still limited. No unified testing protocol is available for their evaluation of microbiological models. Moreover, in most available literature studies, the number of released NPs from cement-based composites is only quantitatively and qualitatively evaluated. Only a few studies dealt with the influence of nanoparticles on microorganisms with a focus on their physiological characteristics, even though they were found to be an excellent indicator for initial screening of the potentially harmful effects of NPs (Coutand et al. 2011; Holden et al. 2014; Sikora et al. 2018a; Augustyniak et al. 2020b).
Therefore, we aimed to evaluate the release of ZnO nanoparticles from cement mortars during different weathering scenarios in the scope of these materials’ physicochemical properties and their influence on model microorganisms’ selected physiological features.
Materials and methods
Materials
Ordinary Portland Cement (OPC) CEM I 42.5R, standard quartz sand (< 2 mm), and tap water conforming European Standards EN 197–1, EN 196–1, and EN 1008 were used (respectively) to produce cement mortars. Chemical composition of cement is presented in Table 1.
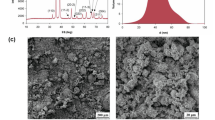
Zinc oxide (ZnO) nanoparticles (CAS Number: 1314-13-2) were obtained from Sigma Aldrich (Darmstadt, Germany). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) nanoparticle shape analysis showed that they have a uniformed size with shape changing from nanorods to spherical nanostructures (Fig. 1a-c). According to the X-ray diffraction (XRD) analysis of obtained nanomaterials are composed of a pure phase of zinc oxide (standard card JCPDS 43–1071) (Fig. 1d). The size of the nanostructures ranged from 50 to 300 nm. According to the MultiPoint BET method, ZnO nanoparticles had a surface area of around 14.11 m2/g.
In total, three bacterial strains were used in the experiments, including two Gram-negative Escherichia coli ATCC®8739™, Pseudomonas aeruginosa PAO1, and P. aeruginosa ATCC®27,583™ and two Gram-positive—Staphylococcus aureus FRI137, S. aureus ATCC®6538™—and a yeast, i.e., Candida albicans ATCC®10,231™. All microorganisms used in this study are widely known opportunistic pathogens.
Mixture composition and mixing process
Mortars with water–cement ratio (w/c) equal to 0.5 were produced conforming to the EN 196–1. The ratio of cement-to-sand-to-water was set to 1:3:0.5. Overall, three types of cement mortars were produced. Control (plain) mortar was designated as C, while mortars containing the addition of 1% and 3% (by weight of cement) of ZnO nanoparticles were designated as 1Z and 3Z, respectively. Incorporating ZnO nanoparticles to the cement mortars was performed with the most common dispersion procedure shown in Fig. 2.
After mixing, the fresh mortar was cast in 40 × 40 × 40 mm3 molds for the first 24 h, and then the mortar prisms were sealed in plastic bags in a temperature-controlled room at 20 ℃ for up to 28 days.
Experimental design
Six different releasing scenarios were proposed. The first two scenarios are standard leaching of mortar cubes at different ages of specimens: 7 and 28 days of curing. Afterward, four different deterioration scenarios were simulated: crushed specimens, abraded specimens (powder obtained from abrasion of specimens), damaged specimens after exposure to the elevated temperature of 600 ℃, and 800 ℃. Before the samples were subjected to thermal treatment in a muffle furnace, specimens were dried overnight at 60 ℃ to remove moisture. Afterwards, specimens were transferred to a muffle furnace and exposed to elevated temperatures of 600 ℃ and 800 ℃ for 1 h, with a heating rate of 1 ℃/min. After heating, samples were cooled at an approximate cooling rate of 1 ℃/min. The experimental plan (outline) of the research is summarized in Fig. 3.
Preparation of samples
Before microbiological experiments, basic engineering properties of cement mortars were evaluated, including consistency and compressive strength. Consistency of fresh mortar was determined using flow-table method following EN 1015–3 standard. Compressive strength of 40 × 40 × 40 mm3 specimens was determined after 28 and 90 days of curing.
The leaching test study (liquid–solid extractions) was performed for 72 h (3 days) at a mass liquid-to-solid ratio (L/S) of ten with ultrapure water (UPW) at room temperature. The three types of hardened cement mortars (C, 1Z, and 3Z) were leached in each leaching scenario.
The leaching procedure was carried out according to EN 12,457–4 and TANK NEN 7375 with modifications since, to our knowledge, no available method covers all types of the tested samples. Standard EN 12,457–4 only refers to comminuted samples with particles of size below 10 mm leached for 24 h (in our research crushed and abraded specimens), while TANK NEN 7375 is dedicated to monolithic structures leached for long periods (samples after different times of curing or treated with 600 ℃ and 800 ℃). Therefore, we introduced a leaching method partially covering both standards to unify all the tested samples’ procedure. After the preparation, the samples were placed on a roller table (30 rpm) for 72 h to obtain eluates. The samples were collected after 30 min of sedimentation by a sterile syringe. In the next step, eluates were filtered by a syringe filter with pore size 0.45 µm and stored in sterile Falcon tubes. After that, the pH measurements of the samples were conducted. For tests including microorganisms, the high pH (around 11–12, depending on a sample) of the leachates had to be altered to the values 6–8 using 0.1 M HNO3 to create the environment enabling the growth of the selected microorganisms.
Physicochemical evaluation of nanomaterials and eluates
The used ZnO nanoparticles were characterized using transmission electron microscopy (TEM—Fei, Tecnai G2 F20 S Twin with energy-dispersive X-ray spectroscopy, Thermo Fisher Scientific, Waltham, MA, USA), scanning electron microscopy (SEM—Tescan Vega 3, Czech Republic), and X-ray diffraction (XRD—X’Pert PRO Philips diffractometer, Co. Ka radiation, Almelo, Holland) as previously described (Sikora et al. 2018a). The evaluation was repeated for this study, and the results are presented in Fig. 1.
Inductively coupled plasma optical emission spectrometer (ICP-OES) was employed to analyze the ion composition in the eluates obtained from specimens. The solution samples were measured with iCAP 6300 ICP Spectrometer (Thermo Scientific, Bremen, Germany). Each ICP-MS measurement was a mean of three results, with each result collected as an average value from 100 measurements. Atomic Absorption Spectrometry (AAS) was performed to characterize the Zn ion content in the specimen using an iCE 3500 spectrometer (Thermo Scientific, Bremen, Germany) with a Zn detection limit of 0.0033 ppm. Samples were vacuum filtered with a 0.45-μm PTFE filter and stabilized with hydrochloric acid to a pH of 2 for ICP-OES and AAS. All results were corrected to the blank value.
Additionally, to visually confirm ZnO nanoparticles’ presence in the obtained eluates were analyzed with a transmission electron microscope with accelerating voltage of 200 kV equipped with the X-ray energy-dispersive spectroscopy (EDS) for elemental analysis. The TEM and EDS samples were suspended in acetone and evaporated at 50 ℃ on the TEM grid with a lacey carbon film. The results from this part are presented in the Supplementary material in Figures S1 and S2.
Microbiological tests
Growth kinetics
The experiment was carried out on a 24-well polystyrene plate with a flat bottom. Each well was filled with 900 µL of overnight culture inoculated to TSB medium (1:400) and 100 µL of the tested eluate. The optical density (λ = 600 nm) measurements were carried out every 30 min, for 8–14 h, depending on the microorganism. Positive and negative controls containing TSB or ultrapure water were included. The experiment was carried out in three replicates. Obtained results were averaged and used to create growth curves that enabled the identification of the logarithmic phase. After that, the µ parameter (growth rate) was calculated according to Tsuchiya et al. (2018), and expressed as a parameter of the ratio of the growth rate of the tested samples to the control growth curve.
4-h toxicity test
The samples for a 4-h toxicity test were selected based on the results from the growth kinetics assay. Only eluates that showed the growth rate parameters lower than 0.9 and greater than 1.1 compared to the control were selected for further testing on a flow cytometer.
The 4-h toxicity test was performed by the dilution (in ratio 1:1) of the chosen microorganism’s overnight culture in phosphate buffer (0.3 mM KH2PO4). The wells of the 96-well polystyrene flat plate were filled with 90 µL of the tested leachate and 10 µL of the prepared diluted culture. The diluted culture with 90 µL of the phosphate buffer served as a positive control, and 100 µL of phosphate buffer was a negative control. The samples were incubated at room temperature for 4 h on an orbital shaker (100 rpm) without access to the light. According to the user’s manual, the preparation of the samples for flow cytometry assay was carried out with BD™ cell viability kit (BD Biosciences, San Jose, CA, USA). Ten thousand events were recorded in each sample. Data analysis was performed with BD Accuri C6 Plus software (BD Biosciences, San Jose, CA, USA).
24-h toxicity test
Overnight cultures of the tested strains were inoculated to TSB medium in ratio 1:400 and added in a volume of 90 µL to a 96-well transparent polystyrene plate with a flat bottom. Each well was filled with 10 µL of each tested eluate. Wells containing a microbe culture in TSB and ultrapure water served as a positive control, whereas fresh TSB medium with ultrapure water was a negative control. Control samples consisting of TSB medium with tested eluate were prepared. The optical density measurements (λ = 600 nm) were carried out immediately after the experiment’s preparation and after 24 h of incubation. The experiment was run in triplicate.
Biofilm formation assay
The experiment was conducted in 96-well transparent polystyrene plate with a rounded bottom. Two biofilming strains of microorganisms, i.e., S. aureus ATCC®6538™ and P. aeruginosa ATCC®27,583™, were selected. The experiment was performed as described in our previous publication (Sikora et al. 2018a) with the exception that all measurements were recorded on BioTek Synergy H1 (BioTek Instruments, Winooski, VT, USA).
Statistical analysis
The results were analyzed statistically with Statistica 13.3 TIBCO Software Inc. (StatSoft Inc., Tulsa, OK, USA) using one-way ANOVA with Tukey test. Differences were considered significant at p < 0.05. All error bars shown in the figures in this manuscript represent standard deviation.
Results and discussion
Consistency and compressive strength of mortars
The flow of fresh mortars (mean diameter) was 145 mm, 143 mm, and 140 mm for C, 1Z, and 3Z mortars, respectively. Therefore, it can be concluded that ZnO nanoparticles have a rather insignificant effect on the consistency of mortars. This phenomenon was most probably attributed to ZnO particles relatively lower specific surface area (14.11 m2/g) in comparison to other widely used nanosized admixtures such as SiO2 or Al2O3 which specific surface area is usually > 100 m2/g (Sikora et al. 2018a; Alhawat et al. 2020). Therefore, in our study, the decrement of consistency was minor.
Figure 4 presents the results of compressive strength determined after 28 and 90 days of curing. After 28 days, the compressive strength of 1Z and 3Z was 24% and 60% lower than that of the control specimen (C). After 90 days of curing, specimen 1Z exhibited comparable strength to that of the C specimen, while the strength of specimen 3Z was one-half of that of C. These observations are in line with the previous showing that low dosages of ZnO usually not exceeding 1 wt.-% (Senff et al. 2014; Liu et al. 2019) are delaying the hydration process significantly. Thus, the strength development in the first weeks of hydration is impeded. However, at later testing ages, specimens exhibit satisfactory mechanical performance (Shafeek et al. 2020). The calorimetric study held by Siler et al. (2019) showed that in OPC system, induction period length increased from 3.3 to 97.4 h when 1 wt.-% of ZnO nanoparticles was incorporated into the cementitious system.
Similarly, Shafeek et al. (2020) showed that the initial setting and final setting were delayed by 38 h and 55 h, respectively, when 1 wt.-% of ZnO nanoparticles was incorporated into the cement. Like our study, other researchers confirmed that higher dosages of ZnO nanoparticles can contribute to the decrement of composites’ strength (Thangapandi et al. 2020). Briefly, the retardation effects of ZnO nanoparticles on the OPC hydration process can be attributed to the fact of ZnO presence in the cement mix resulting in the formation of CaZn2(OH)6·2H2O and Zn(OH)2 layers of around anhydrous particles of cement at the beginning of the hydration reaction, thus preventing water from reacting to form the tricalcium silicate (C3S) phase. This phenomenon has been extensively studied and described in Shafeek et al. (2020).
Physicochemical evaluation of nanomaterials and eluates
Both spectroscopic methods confirmed Zn’s presence in filtered leachates of the samples with the addition of 3% Zn (all samples besides 28-day-old blocks) and abraded 1% sample. It indicates that the samples’ syringe filtering did not cause the gathering of all the nanoparticles on the filter surface. However, filtering might reduce the nanomaterial concentration, e.g., abraded samples clogged the filter pores relatively quickly. On the other hand, the filtering process allowed the separation of the unwanted residues and agglomerates and was used by other authors working with cement-based eluates (Coutand et al. 2011; Bossa et al. 2017).
Table 2 presents the Zn content results in the eluates determined both by ICP-OES and AAS techniques. Like other studies, discrepancies between the results obtained from both techniques can be reported (Jarošová et al. 2014; Mohammed et al. 2016). In our study, the Zn content determined with AAS was generally lower or below the detection rate, while in the case of ICP-OES, Zn presence was detected in all tested specimens. In general, zinc is present in cement clinker. Thus, its presence can be detected using different evaluation techniques (Lu et al. 2016; Mocová et al. 2019). However, as Lu et al. (2016) reported, Zn is usually immobilized within the cement matrix; thus, very low Zn contents are detected.
Nevertheless, two main conclusions from the data obtained in our study can be derived. First, a gradual increment of Zn in eluates and the increment of the ZnO nanoparticles dosage can be reported. Second, higher Zn presence was reported in specimens that underwent various deterioration processes. The highest amount of Zn released was reported in specimens 3ZC followed by 3Z800. As it was reported by Lu et al. (2016), the leaching of metals is affected by sizes of particles of solid waste, with more rapid dissolution occurring for that finer particle size (larger surface areas per mass of volume). Therefore, higher Zn content was leached from crushed specimens than solid mortar blocks.
Moreover, initial exposure of specimens to elevated temperature (600 ℃ and 800 ℃) resulted in the induction of the cracks in specimens and increment of the specimens’ porosity (Sikora et al. 2018b), thus after water immersion, specimens had a higher tendency to Zn release. Surprisingly, a relatively low release rate was reported in abraded specimens when compared to crushed specimens. It could be attributed to the limitation of the filtration efficiency of highly fine powder. Moreover, the lower amount of Zn released was reported in 28-day series than 7-day series which confirms that the release rate depends on the hydration degree (higher porosity of the 7 days specimens than 28 days).
Microbiological tests
Growth kinetics
To assess changes in the growth rates of the chosen microorganisms, it was proposed to introduce a parameter characterizing the ratio of the growth rate in the tested samples to the growth rate of the control samples (µsample/µcontrol) (Fig. 5,6) (Tsuchiya et al. 2018). E. coli incubated with 8 out of 18 elutes obtained the growth parameter greater than 1.1. On the other hand, all S. aureus results comprised the values of 0.9–1.1. P. aeruginosa growth parameters after incubation with eluates C7 and 3Z7 equaled 1.11 and 1.26, respectively. C. albicans obtained parameters equal to 1.17 (C7), 0.84 (3ZA), 0.87 (3Z600) and 0.87 (1Z800). Obtained results suggest that eluate from the sample 3ZA (abraded with 3% of ZnO) had a substantial impact on the growth rate of P. aeruginosa and C. albicans as the calculated parameters were lower than 0.85. However, E. coli incubated with the same leachate obtained the growth parameter equal to 1.14. These results indicated that eluates could change the growth rate in tested microorganisms, similar to results obtained in our previous work (Sikora et al. 2018a).
4-h toxicity test
The growth kinetics analysis revealed that some samples caused a difference in the growth rate from the control for E. coli, P. aeruginosa, and C. albicans. These materials were selected for a 4-h toxicity test. The results have shown that most of these materials had a relatively low effect on the tested cells; however, there were some exceptions. Samples 3Z7 for C. albicans and 3Z600 and 1Z800 have increased the percentage of dead cells in the studied samples. These results are following the results obtained in the 24-h toxicity test.
On the other hand, the increased toxicity of sample 3Z7 detected in P. aeruginosa contradicts a stimulation effect obtained in growth rate analysis. It could be accounted for the lack of nutrients in the 4-h test, and the bacterium had limited resources to defend cells from the environmental stressors (Yang et al. 2012; van Kessel 2019). In the case of E. coli, several samples caused a slightly increased death rate that could be accidental because of the low differences between samples.
24-h toxicity test
In the experiments concerning 7-day-old samples, the noticeable rise of OD (Fig. 7) was noted for 3% (3Z) samples with S. aureus and 1% (1Z) samples with P. aeruginosa and C. albicans. However, a significant decrease in OD value was observed in all tests with E. coli, for the reference sample (C) and 3% sample with P. aeruginosa. In most of the tests (except one case in P. aeruginosa), the viability of cells decreased or was at the control sample level.
The experiments with 28-day-old samples (Fig. 8) showed an increase in the OD for all tests with P. aeruginosa, 3% sample with E. coli, and 1% sample with C. albicans. The OD decrease was noted for control and 1% sample with S. aureus. In all tests, the viability of cells decreased or was on the level of the control sample.
The OD of abraded samples (Fig. 9) with E. coli and S. aureus was lower than in the control sample. For C. albicans, the OD lowered only with a 3% sample (3ZA). However, tests with P. aeruginosa scored higher OD values. The viability of cells was comparable (for S. aureus and C. albicans) or lower than the control sample (for E. coli and P. aeruginosa).
In tests with crushed samples (Fig. 8), the decrease in OD was noted for 3% sample (3ZC) with S. aureus and 1% (1ZC) and 3% samples with C. albicans. On the other hand, experiments with P. aeruginosa showed an increase in OD. Cell viability was higher than the control only for S. aureus tested with 1% and 3% samples.
The results of the tests of the blocks treated with 600 ℃ (Fig. 10) demonstrated the OD increase for P. aeruginosa analyses, the decrease for E. coli, and no significant changes of OD value for S. aureus and C. albicans. The cell viability was higher for E. coli and C. albicans tested with reference eluate (C600), but a significant decrease was noted for all samples with P. aeruginosa.
The analyses with blocks treated with 800 ℃ (Fig. 10) showed higher OD values for all samples with P. aeruginosa and 3% samples with S. aureus. The decrease of OD was noted for E. coli. The viability of cells was decreased for E. coli with 1% sample and all samples with P. aeruginosa. Simultaneously, the rest of the eluates exhibited no significant differences in the number of viable cells.
The cell viability, in most cases, was decreased or equal to the control values. The only exceptions were samples that were crushed (1ZC and 3ZC) in the test with P. aeruginosa, control samples treated with 600 ℃ (C600) with E. coli and C. albicans, and 3% Zn samples (3Z28 and 3Z800 in biofilm assay).
The gained results indicated that the addition of ZnO nanoparticles into the cement mortars and their subsequent release could affect tested microorganisms’ viability, as was indicated in our previous work (Sikora et al. 2018a). However, some mild effects from cement mortars cannot be excluded since these materials are not neutral to microorganisms because of the pH (which was adjusted in this case) and high load of minerals (Sikora et al. 2016). It is in accordance with observations by Rodrigues et al. (2020), who also detected some mild toxicity in their samples (Rodrigues et al. 2020).
Biofilm formation assay
The outcome of the biofilm assay (Fig. 11) for S. aureus was that 3% 7-day-old sample, all samples treated with 600 ℃, 3% samples treated with 800 ℃, abraded and crushed samples (reference and 1% variant) showed significantly lower biofilm biomass than the control sample. The lowest cell viability was noted for abraded samples, especially 3ZA. However, 3% of the 28-day-old sample and 3% sample treated with 800 °C were identified as much higher cell viability than the control one.
Pseudomonas aeruginosa biofilm biomass was greater than the control biomass in most tested samples, while the cell viability decreased. The stimulation of biofilm formation can trigger this bacterium to respond to unfavorable conditions (Rasamiravaka et al. 2015; Chen et al. 2018). On the other hand, the experiment with 3Z800 samples showed cell viability on the control level, and no significant biomass increase. Moreover, S. aureus incubated with the same sample showed the decreased biofilm biomass but higher viability. It may indicate the lower toxicity of the sample mentioned above (Sikora et al. 2018a).
P. aeruginosa biofilm assay (Fig. 12) showed that for most of the tested samples, the biofilm biomass was higher than the control. Nevertheless, the cell viability was decreased for abraded samples, reference (CC), and 1% crushed (1ZC) blocks.
Obtained results did not indicate the toxicity at level observed in previous studies since the obtained concentrations of the nanomaterial was relatively low when compared to the amounts of ZnO NPs that were reported to be toxic to microorganisms by other authors (Kumar et al. 2011b; Bondarenko et al. 2013; Sikora et al. 2018a). Furthermore, the ecotoxicity of the cement mortar leachates has been previously reported relating to the aquatic organisms, e.g., Daphnia magna, duckweed, or Vibrio fischeri, and the focus was put on the assessment of the variables such as mobility, many fronds, and chlorophyll content or luminescence, respectively (Brás et al. 2017; Bandow et al. 2018). However, this study’s objects were model we focused on assessing the parameters such us growth kinetics, viability, and biofilm formation to investigate the effect of the eluates on the physiology of the selected reference bacteria and yeast that are considered opportunistic pathogens. This approach allowed us to detect the effects of cement mortar leachates containing and lacking the ZnO NPs on their physiology. Our studies confirmed that the effect may depend on the strain and environment (Sikora et al. 2018a). In this research, we have shown that in the favorable environment, some eluates could even promote the growth of selected microorganisms (Fig. 5) or their biofilm formation (Fig. 12), even though the viability of cells in the biofilm could be lower. The detected physiological changes are alarming, because shifts in the growth rate, metabolic activity and biofilm formation caused by environmental stressors may have an impact on the virulence of tested bacteria (Ezraty et al. 2017).
Conclusion
Our studies have shown that the highest amount and influence of released ZnO nanoparticles on microorganisms were noticed for the samples with the highest ZnO ratio and exposed to weathering scenarios. After exposure to high temperatures, ZnO nanoparticles’ release was foreseeable due to the significant decrease in cement stability and disintegration. However, our study also confirmed that mechanical abrasion also resulted in the release of a certain amount of ZnO nanoparticles. The obtained eluates have shown relatively low effectiveness against the tested microorganisms with the released nanoparticles’ low concentration. Nevertheless, the release of ZnO from abraded and crushed cement mortars was enough in some cases to affect the physiology of model microorganisms, including their viability and biofilm formation ability. The described changes may have an impact on their virulence; however, the direct influence of nanomaterials on the bacterial virulence is yet to be determined in further studies and on selected cases. It should be also emphasized that our tests were focused on filtered solutions where the tested nanoparticulate components of the tested cement mortars were separated from the bigger particles. It is clear that the degradation of cement mortars cause changes in the surrounding environment which are probably not neutral to the microorganisms inhabiting adjacent niche.
References
Alhawat M, Ashour A, El-Khoja A (2020) Properties of concrete incorporating different nano silica particles. Mater Res Innov 24(3):133–144. https://doi.org/10.1080/14328917.2019.1616140
Arefi MR, Rezaei-Zarchi S (2012) Synthesis of zinc oxide nanoparticles and their effect on the compressive strength and setting time of self-compacted concrete paste as cementitious composites. Int J Mol Sci 13:4340–4350. https://doi.org/10.3390/ijms13044340
Augustyniak A, Cendrowski K, Nawrotek P et al (2016) Investigating the interaction between Streptomyces sp. and Titania/Silica Nanospheres. Water Air Soil Pollut 230:1–13. https://doi.org/10.1007/s11270-016-2922-z
Augustyniak A, Sikora P, Cendrowski K et al (2019) Challenges in studying the incorporation of nanomaterials to building materials on microbiological models. In: Fesenko O, Yatsenko L (eds) Nanophotonics, nanooptics, nanobiotechnology, and their applications. Springer Nature, Switzerland, pp 285–303
Augustyniak A, Cendrowski K, Grygorcewicz B et al (2020a) The response of pseudomonas aeruginosa pao1 to uv-activated titanium dioxide/silica nanotubes. Int J Mol Sci. https://doi.org/10.3390/ijms21207748
Augustyniak A, Sikora P, Jablonska J et al (2020b) The effects of calcium–silicate–hydrate (C–S–H) seeds on reference microorganisms. Appl Nanosci. https://doi.org/10.1007/s13204-020-01347-5
Bandow N, Gartiser S, Ilvonen O, Schoknecht U (2018) Evaluation of the impact of construction products on the environment by leaching of possibly hazardous substances. Environ Sci Eur 30:14
Bondarenko O, Juganson K, Ivask A et al (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200
Bossa N, Chaurand P, Levard C et al (2017) Environmental exposure to TiO2 nanomaterials incorporated in building material. Environ Pollut 220:1160–1170. https://doi.org/10.1016/j.envpol.2016.11.019
Bossa N, Chaurand P, Levard C et al (2019) Mechanisms limiting the release of TiO 2 nanomaterials during photocatalytic cement alteration: the role of surface charge and porous network morphology. Environ Sci Nano 6:624–634. https://doi.org/10.1039/c8en00700d
Brás I, Silva PC, Almeida R, et al (2017) Eco-toxicity assessment of concrete prepared with industrial wastes. In: Energy Procedia. Elsevier Ltd, pp 115–120
Bräu M, Ma-Hock L, Hesse C et al (2012) Nanostructured calcium silicate hydrate seeds accelerate concrete hardening: a combined assessment of benefits and risks. Arch Toxicol 86:1077–1087. https://doi.org/10.1007/s00204-012-0839-x
Butler KS, Casey BJ, Garborcauskas GVM et al (2014) Assessment of titanium dioxide nanoparticle effects in bacteria: Association, uptake, mutagenicity, co-mutagenicity and DNA repair inhibition. Mutat Res - Genet Toxicol Environ Mutagen 768:14–22. https://doi.org/10.1016/j.mrgentox.2014.04.008
Chen H, Wubbolts RW, Haagsman HP, Veldhuizen EJA (2018) Inhibition and eradication of pseudomonas aeruginosa biofilms by host defence peptides. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-28842-8
Coutand M, Cyr M, Clastres P (2011) Quantification of uncertainty of experimental measurement in leaching test on cement-based materials. J Environ Manage 92:2494–2503. https://doi.org/10.1016/j.jenvman.2011.05.011
Diamond SA, Kennedy AJ, Melby NL et al (2017) Assessment of the potential hazard of nano-scale TiO2 in photocatalytic cement: application of a tiered assessment framework. NanoImpact 8:11–19. https://doi.org/10.1016/j.impact.2017.06.006
Elia HN, Nima ZA (2019) Self-cleaning concrete doped with nano and micro-size zinc oxide particles. Int J Eng Res Dev 15:17–22
Ezraty B, Gennaris A, Barras F, Collet JF (2017) Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15:385–396. https://doi.org/10.1038/nrmicro.2017.26
Funk B, Göhler D, Sachsenhauser B et al (2019) Impact of freeze-thaw weathering on integrity, internal structure and particle release from micro- and nanostructured cement composites. Environ Sci Nano 6:1443–1456. https://doi.org/10.1039/c8en01397g
Ganguly P, Breen A, Pillai SC (2018) Toxicity of Nanomaterials: exposure, pathways, assessment, and recent advances. ACS Biomater Sci Eng 4:2237–2275. https://doi.org/10.1021/acsbiomaterials.8b00068
Ge Y, Schimel JP, Holden P, a, (2011) Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol 45:1659–1664. https://doi.org/10.1021/es103040t
Ghafari E, Ghahari SA, Feng Y et al (2016) Effect of Zinc oxide and Al-Zinc oxide nanoparticles on the rheological properties of cement paste. Compos Part B Eng 105:160–166. https://doi.org/10.1016/j.compositesb.2016.08.040
Ghahari SA, Ghafari E, Lu N (2017) Effect of ZnO nanoparticles on thermoelectric properties of cement composite for waste heat harvesting. Constr Build Mater 146:755–763. https://doi.org/10.1016/j.conbuildmat.2017.04.165
Giese B, Klaessig F, Park B et al (2018) Risks, release and concentrations of engineered nanomaterial in the environment. Sci Rep 8:1565. https://doi.org/10.1038/s41598-018-19275-4
Holden PA, Schimel JP, Godwin HA (2014) Five reasons to use bacteria when assessing manufactured nanomaterial environmental hazards and fates. Curr Opin Biotechnol 27:73–78. https://doi.org/10.1016/j.copbio.2013.11.008
Jarošová M, Milde D, Kuba M (2014) Elemental analysis of coffee. a comparison of ICP-MS and AAS methods. Czech J Food Sci 32:354–359. https://doi.org/10.17221/399/2013-cjfs
Jones W, Gibb A, Goodier C et al (2016) Nanomaterials in construction–what is being used, and where? Proc Inst Civ Eng Mater. https://doi.org/10.1680/jcoma.16.00011
Kamal HM, Kadhim MJ, Jawad RKM (2020) Investigate the colloidal nano-zinc oxide addition on the strength acceleration of G-sand cement mortar. AIP Conf Proc. https://doi.org/10.1063/5.0000294
Kumar A, Pandey AK, Singh SS et al (2011a) A flow cytometric method to assess nanoparticle uptake in bacteria. Cytom Part A 79:707–712. https://doi.org/10.1002/cyto.a.21085
Kumar A, Pandey AK, Singh SS et al (2011b) Engineered ZnO and TiO 2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic Biol Med 51:1872–1881. https://doi.org/10.1016/j.freeradbiomed.2011.08.025
Le Pivert M, Poupart R, Capochichi-Gnambodoe M et al (2019) Direct growth of ZnO nanowires on civil engineering materials: smart materials for supported photodegradation. Microsystems Nanoeng 5:1–7. https://doi.org/10.1038/s41378-019-0102-1
Lee J, Mahendra S, Alvarez PJJ (2010) Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano 4:3580–3590. https://doi.org/10.1021/nn100866w
Liu J, Jin H, Gu C, Yang Y (2019) Effects of zinc oxide nanoparticles on early-age hydration and the mechanical properties of cement paste. Constr Build Mater 217:352–362. https://doi.org/10.1016/j.conbuildmat.2019.05.027
Lu H, Wei F, Tang J, Giesy JP (2016) Leaching of metals from cement under simulated environmental conditions. J Environ Manage 169:319–327. https://doi.org/10.1016/j.jenvman.2015.12.008
Mielcarz-Skalska L, Smolińska B (2017) Zinc and nano-ZnO – influence on living organisms. Biotechnol Food Sci 81:93–102
Miller HD, Mesgari S, Akbarnezhad A, Foster S (2017) Safety risks associated with carbon nanotube-reinforced mortar. ACI Mater J 114:897–909
Mitrano DM, Motellier S, Clavaguera S, Nowack B (2015) Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int 77:132–147. https://doi.org/10.1016/j.envint.2015.01.013
Mocová KA, Sackey LNA, Renkerová P (2019) Environmental impact of concrete and concrete-based construction waste leachates. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/290/1/012023
Mohammed H, Sadeek S, Mahmoud AR, Zaky D (2016) Comparison of AAS, EDXRF, ICP-MS and INAA performance for determination of selected heavy metals in HFO ashes. Microchem J 128:1–6. https://doi.org/10.1016/j.microc.2016.04.002
Nazari A, Riahi S (2011) The effects of ZnO2 nanoparticles on strength assessments and water permeability of concrete in different curing media. Mater Res 14:178–188. https://doi.org/10.1590/S1516-14392011005000030
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22. https://doi.org/10.1016/j.envpol.2007.06.006
Osman DAM, Nur O, Mustafa MA (2020) Reduction of energy consumption in cement industry using zinc oxide nanoparticles. J Mater Civ Eng 32:04020124. https://doi.org/10.1061/(asce)mt.1943-5533.0003196
Philippot L, Spor A, Hénault C et al (2013) Loss in microbial diversity affects nitrogen cycling in soil. ISME J 7:1609–1619. https://doi.org/10.1038/ismej.2013.34
Rasamiravaka T, Labtani Q, Duez P, El Jaziri M (2015) The formation of biofilms by pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int. https://doi.org/10.1155/2015/759348
Rodrigues P, Silvestre JD, Flores-Colen I et al (2020) Evaluation of the ecotoxicological potential of fly ash and recycled concrete aggregates use in concrete. Appl Sci 10:351. https://doi.org/10.3390/app10010351
Senff L, Tobaldi DM, Lemes-Rachadel P et al (2014) The influence of TiO2 and ZnO powder mixtures on photocatalytic activity and rheological behavior of cement pastes. Constr Build Mater 65:191–200. https://doi.org/10.1016/j.conbuildmat.2014.04.121
Shafeek AM, Khedr MH, El-Dek SI, Shehata N (2020) Influence of ZnO nanoparticle ratio and size on mechanical properties and whiteness of White Portland Cement. Appl Nanosci 10:3603–3615. https://doi.org/10.1007/s13204-020-01444-5
Sikora P, Augustyniak A, Cendrowski K et al (2016) Characterization of mechanical and bactericidal properties of cement mortars containing waste glass aggregate and nanomaterials. Materials (Basel) 9:1–16. https://doi.org/10.3390/ma9080701
Sikora P, Augustyniak A, Cendrowski K et al (2018a) Antimicrobial activity of Al2O3, CuO, Fe3O4, and ZnO nanoparticles in scope of their further application in cement-based building materials. Nanomaterials 8:212. https://doi.org/10.3390/nano8040212
Sikora P, Cendrowski K, Horszczaruk E, Mijowska E (2018b) The effects of Fe3O4 and Fe3O4/SiO2 nanoparticles on the mechanical properties of cement mortars exposed to elevated temperatures. Constr Build Mater 182:441–450. https://doi.org/10.1016/j.conbuildmat.2018.06.133
Siler P, Kolarova I, Bednarek J et al (2019) The effect of zinc, water to binder ratio and silica fume on the hydration and mechanical properties of Portland cement mixtures. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/583/1/012008
Silva F, Arezes P, Swuste P (2018) Risk management: Controlling occupational exposure to nanoparticles in construction. Elsevier Ltd, Newyork
Spinazzè A, Cattaneo A, Limonta M et al (2016) Titanium dioxide nanoparticles: occupational exposure assessment in the photocatalytic paving production. J Nanoparticle Res. https://doi.org/10.1007/s11051-016-3462-6
Strokova V, Nelubova VV, Sivalneva M, Kobzev V (2018) Phytotoxicity analysis of different compositions of nanostructured binder. Key Eng Mater 761:189–192. https://doi.org/10.4028/www.scientific.net/KEM.761.189
Thangapandi K, Anuradha R, Archana N et al (2020) Experimental study on performance of hardened concrete using nano materials. KSCE J Civ Eng 24:596–602. https://doi.org/10.1007/s12205-020-0871-y
Torres-Carrasco M, Reinosa JJ, de la Rubia MA et al (2019) Critical aspects in the handling of reactive silica in cementitious materials: effectiveness of rice husk ash vs. nano-silica in mortar dosage. Constr Build Mater 223:360–367. https://doi.org/10.1016/j.conbuildmat.2019.07.023
Tsuchiya K, Cao YY, Kurokawa M et al (2018) A decay effect of the growth rate associated with genome reduction in Escherichia coli. BMC Microbiol 18(101):1–10. https://doi.org/10.1186/s12866-018-1242-4
Van Broekhuizen P, Van Broekhuizen F, Cornelissen R, Reijnders L (2011) Use of nanomaterials in the European construction industry and some occupational health aspects thereof. J Nanoparticle Res 13:447–462. https://doi.org/10.1007/s11051-010-0195-9
van Kessel JC (2019) PQS signaling for more than a quorum: the collective stress response protects healthy pseudomonas aeruginosa populations. J Bacteriol. https://doi.org/10.1128/JB.00568-19
Yang Y, Mathieu JM, Chattopadhyay S et al (2012) Defense mechanisms of Pseudomonas aeruginosa PAO1 against quantum dots and their released heavy metals. ACS Nano 6:6091–6098. https://doi.org/10.1021/nn3011619
Acknowledgements
This research was funded by the National Science Centre (Poland) within project No. 2016/21/N/ST8/00095 (PRELUDIUM 11). Adrian Augustyniak and Dietmar Stephan were supported by the German Research Foundation (DFG) as part of the Research Training Group on Urban Water Interfaces (GRK 2032).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Submitted to special issue NANO-2020 within the 8th International Conference “Nanotechnologies and Nanomaterials” NANO-2020.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Augustyniak, A., Jablonska, J., Cendrowski, K. et al. Investigating the release of ZnO nanoparticles from cement mortars on microbiological models. Appl Nanosci 12, 489–502 (2022). https://doi.org/10.1007/s13204-021-01695-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01695-w