Abstract
The science and technology of catalysis is of fundamental importance to a national economy. Today about 90% of all technical chemicals are manufactured by the use of catalysts. Nanoparticles of noble metals are extremely important materials in the catalysis industry due to cost issues and properties that are not found in their bulk state. An efficient way to produce and stabilise noble metal nanoparticles is by dispersion on a suitable support. Carbon-based supports, such as carbon nanotubes, carbon spheres, carbon fibres, etc., have been found to be good supports for metal nanoparticles. However, to be used effectively, the carbon surface must be modified either by functionalisation or doping. This review discusses the synthesis and the possible applications of nitrogen-doped carbon nanotubes as supports for metal nanoparticles in heterogeneous catalysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catalysis plays an important role in the production of many compounds in numerous fields such as fuels and fine chemicals (Ledoux and Pham-Huu 2005; Reddy et al.2006). A key issue in catalysis is to obtain high surface area catalysts in order to enhance catalytic activity and selectivity (Ledoux and Pham-Huu 2005; Gao et al.2006). This is achieved by using support oxides of the transition metals, such as silicon dioxide, titanium dioxide, etc., which are among the most commonly used heterogeneous catalyst supports (Martínez-Méndez et al.2006). Catalyst activity depends on the particle size and appropriate distance between each particle. These catalysts deposited on a support are affected by the surface reactivity and the microstructure of the support (Chakrabarty 1990; Ledoux and Pham-Huu 2005). The surface area of the support can be increased by exposing the support to corrosive treatment to develop cracks and pores. The higher the surface area of the support the better the dispersion of the catalyst on a high surface area material (Chakrabarty 1990). The morphology and the nature of catalyst support is considered to be important factors in obtaining a high dispersion of nanoparticles (Gao et al. 2006).The support can also influence the performance of the catalyst through electronic interactions and migration effects (Mhlanga 2009).
A catalyst support is a material on which an active metal nanoparticle is deposited. It can be granular, fibrous or a monolithic material with high thermal stability. Examples include either naturally occurring materials, such as clays and zeolites or synthetic materials like silica, titania, alumina and zirconia (Chakrabarty 1990). The challenge related to the use of the above supports is that they are not easy to remove from the products at the end of the reaction due to their high degree of insolubility. To overcome this challenge, carbon has been used as a support (Chen et al. 2004, 2010a, b, c; Gao et al. 2006; Garcia et al. 2006; Guczi et al. 2006; Reddy et al. 2006; Chen et al. 2007; Antolini 2009). Carbon can, in principle, be removed by oxidation.
In recent years, different forms of carbon have been used to support many different metals. In particular the use of carbon nanotubes (CNTs) has been extensively reported. To achieve good interaction between CNTs and the metal, CNTs can be functionalised or doped. The most common dopant used to modify the behaviour of CNTs is nitrogen. Doping of CNTs with nitrogen leads to nitrogen-doped CNTs (N-CNTs).
This article critically reviews the work reported to date using N-CNTs as support materials for catalysts.
CNTs
CNTs can be described as an one-dimensional form of a fullerene with a well-defined direction along the nanotube axis that is analogous to the in-plane directions of graphite. CNTs can be either single-walled (SWCNTs), double-walled (DWCNTs), or multi-walled (MWCNTs). Ideally, SWCNTs are made of a perfect graphene sheet, rolled up into a cylinder and closed by two caps (Li and Zhang 2005; Fan et al.2006). The diameters of SWCNTs are typically in a range of 0.4–3 nm, while the length can be up to several microns. Typically, MWCNTs can be considered as a series of concentric SWCNTs with inner diameters of 1–30 nm and with outer diameters of up to 100 nm (Fan et al.2006). Since the report of CNTs by Iijima in 1991, their physical and electronic properties have generated considerable interest. The most noticeable properties are their excellent mechanical toughness, good electrical conductivity, and high tensile strength, which are required for numerous applications (Thostenson et al.2001; Montoro and Rosolen 2006). One of the first observed major commercial applications of MWCNTs was their use as an electrically conducting material with dramatically increased modulus and strength (Dresselhaus et al. 1996). Porous CNT arrays have a high electrochemical accessible surface area and combined with their high electronic conductivity and useful mechanical properties, these materials are attractive as electrodes for devices that use electrochemical double layer charge injection (Fan et al.2006). Therefore, they are used for energy storage, e.g. for fuel cells that power electric vehicles or laptop computers (Yu et al.2000). They can be applied as field emission electron sources, for flat panel displays, lamps and gas discharge tubes providing surge protection and as X-ray and microwave generators (Fan et al.2006).
Nieto-Márquez et al. (2008) revealed that CNTs possess fascinating physical and chemical properties with electronic behaviour varying from metallic to semiconducting depending on their structural composition, chirality and diameter. It is still a challenge to precisely control these parameters in the growth process of the CNTs and hence it is also not easy to control the properties or the reactivity of the CNT outer wall (Tao et al.2007). The CNT surfaces are usually chemically modified by acid or oxidation treatment, which considerably reduces the chemical and electronic performance of the tubes due to the introduction of a large number of defects.
CNTs are attractive supports in heterogeneous catalytic processes owing to their ability to be easily tailored to meet specific needs. They are employed as supports due to their high surface area, resistance to acidic/basic media, stability at high temperatures, and the possibility of controlling both their porous structure and the chemical surface nature (Dai et al.1996; Ebbesen et al.1996; Gao et al.2006). CNTs have been studied as supports for many metal catalysts (Liang et al.2005; Bahome et al.2007). Nanotubes are special because they have small dimensions, smooth surface topology, and perfect surface specificity. CNTs are known to enhance the catalytic performance such that the activity of a catalyst becomes higher than that encountered with traditional catalyst supports in both gas phase and liquid phase reactions (Collins and Avouris 2000).
N-CNTs
The doping of CNTs with heteroatoms offers a practical path to tailor both the physical and chemical properties of the CNTs by creating new states that modify their electronic structure (Panchakarla et al. 2010; Saha and Kundu 2010; Cruz-Silva et al.2008). Boron and nitrogen are among the most effective dopants because of their small atomic size similar to that of the carbon atom. They thus have a reasonable probability of entering a nanotube lattice (Iijima 1991; Villalpando-Paez et al.2006; Sumpter et al.2007; Ghosh et al.2008a; Koós et al.2009). In particular, the substitutional doping of nitrogen has received attention because significant changes in hardness, electrical conductivity, and chemical reactivity of CNTs have been theoretically predicted and experimentally observed in the doped carbon (Maldonado et al.2006). Additional lone pairs of electrons on N atoms with respect to the delocalised π-system of a graphite-like hexagonal framework can enhance the electronic properties of the carbon material (Ghosh et al. 2010; Ajayan 1999; Matter et al.2006). N-CNTs are found to be exclusively metallic conductors or to have a narrow energy gap, thus increasing their storage capacity and offering the possibility of greater electrical conductivity as compared with undoped CNTs (Chizari et al. 2010; Ghosh et al. 2010; Treacy et al.1996; Baughman et al.2002). In most cases, CNTs doped with N exhibit a bamboo-like structure with regularly arranged compartments (refer to Fig. 1).
Nitrogen atoms entering the graphene sheets as substituents for carbon could also modify the adsorption strength of the nanotube towards foreign elements quite significantly, which in turn, greatly modify the overall catalytic activity as well as selectivity of a catalyst (Amadou et al.2008). Generally, nitrogen is incorporated into the CNT lattice during CNT growth. The possible bonding configurations for N in graphitic networks are shown in Fig. 2. The three most common bonding configurations are (a) pyridine-like sp2—hybridized sixfold ring arrangement, (b) pyrrole-like sp2—hybridized fivefold ring arrangement, (c) substitutional sp3—hybridized sixfold ring arrangement, and (d) oxidised pyridinic (Burch 2006; Van Dommele et al.2008).
Types of nitrogen species found in N-CNTs. a pyridinic, b pyrrolic, c quaternary and d oxidised pyridinic. (Van Dommele et al.2008)
N-CNTs are promising activated carbons that demonstrate enhanced chemical reactivity for electron transfer processes (Baughman et al.2002). Although many studies have evaluated the structure-composition-property relationships of these N-doped carbons, the influence of nitrogen doping has not been fully established (Baughman et al.2002).
Synthesis of N-CNTs
In this section we review the methods used to make N-CNTs. The doping of a carbon nanostructured with N can be divided into two types: (1) doping directly during the synthesis of carbon nanostructured materials, which can be called “in situ” doping and (2) post-treatment of CNTs with nitrogen-containing precursors such as N2, NH3, etc., i.e., post doping. The ‘in situ’ doping method is the most common method used to make N-CNTs (Shao et al.2008). Incorporation of foreign atoms into CNTs was first performed by Stephan and co-workers (Stephan et al.1994) who doped CNTs with boron and nitrogen using an arc discharge technique. The arc discharge, chemical vapour deposition (CVD) and laser ablation methods have been employed for the synthesis of CNT and these methods are also suitable for the synthesis of N-CNTs. These techniques produce N-CNTs with regular internal bamboo cavities (refer to Fig. 1) whose growth mechanism is difficult to understand. A recently proposed growth model is depicted in Fig. 3. During synthesis, CNx material is generated and deposited on the metal catalyst particle where they react exothermically. Diffusion of CNx species through the metal catalyst particle is accountable for CNT growth and encapsulation of N2 results from the decomposition of unstable reactive intermediates. The compartments are caused by the different precipitation rates of different CNx species. When precipitation is slow, and there is a lack of material to maintain inner CNT growth, layers will suddenly close (Burch 2006).
Proposed growth mechanism for N-CNTs (Reyes-Reyes et al.2004)
Most methods produce CNTs with by-products such as amorphous carbon that necessitate additional purification steps (Nieto-Márquez et al.2008; Matlhoko et al.2009). The disadvantages of the arc discharge and laser ablation processes are (1) these processes are not very well controlled, nor are they continuous; (2) the removal of impurities with simple, cheap purification methods is difficult; (3) it is also difficult to grow well-aligned nanotubes as they require the use of expensive instrumentation. The CVD method is the most commercially viable technique used to make N-CNTs. CVD method have advantage of high yields and the controllable growth conditions (Li et al.2008)thus reveal that scalability is not a problem. However, a drawback of the CVD process is the production of poor-quality CNTs that contain defects. This happens because the structures are created at much lower temperatures of 600°–1,000°C compared with arc and laser techniques, where the temperatures of the plasmas are greater than 2,000°C. It is likely that the defects are annealed at high temperatures during the growth process (Dresselhaus et al. 2001).
Based on numerous trial-and-error studies published in the literature, there is a clear agreement that the successful introduction of nitrogen atoms within the walls of CNTs depends on the choices of precursor, catalyst, reaction time and temperature, pressure and carrier gas flow rate (Koós et al.2009; Yadav et al.2009). Various studies indicate that ammonia is an effective nitrogen source for the synthesis of N-CNTs. Liu et al. (2005) synthesised vertically aligned N-CNTs while studying the effect of ammonia on the nitrogen content of N-CNTs. These nanotubes were synthesised from the pyrolysis of pyridine and ferrocene as carbon source and catalyst source, respectively. The N was shown to substitute C in the carbon lattice in both pyridinic and graphitic forms. Chun et al. (2009) have also shown that ammonia is an effective N-source for the preparation of N-CNTs achieved by the catalytic decomposition of methane and ammonia over Fe–Mo catalysts. Large amounts of tangled carbon filaments with a length of more than tens of micrometers were obtained. Periodically closed N-CNTs with a nitrogen content up to 7% were synthesised by Amadou and co-workers (Amadou et al.2008) using a mixture of C2H6/H2 and NH3 over an alumina supported Fe catalyst.
When NH3 is used as a N-source, it requires the co-addition of a carbon precursor, and the process was found to be difficult to control (Ghosh et al.2008b). To overcome this difficulty of using ammonia, precursors containing both carbon and nitrogen have been used. Ghosh et al. (Ghosh et al.2008a) obtained highly aligned bamboo-shaped CNTs by the catalytic pyrolysis of monoethanolomine/ferrocene mixtures over the temperature range 700°–900°C by a CVD method. During the deposition, monoethanolomine decomposed to form ammonia, which is very effective for the growth of N-CNTs. MWCNTs containing nitrogen have also been grown by an aerosol CVD using ferrocene/benzylamine solutions. Scanning tunnelling microscopy measurements showed that the local density of states of nanotubes is asymmetrical, with a higher density of states above the Fermi level. Approximately 9.7 wt% nitrogen was determined by energy dispersive spectroscopy (Osváth et al. 2009). Nxumalo et al. (2010) studied the effect of varying the N-source concentration on the production of N-CNTs by a floating catalyst CVD method using a ferrocene/aniline/toluene solution. A ferrocene/aniline catalyst of 15 wt% produced N-CNTs with a small amount of by-products. It was also revealed that the diameters of the tubes can be controlled by varying the N-source concentration. Well-aligned as-grown N-CNT bundles having lengths of about 430 μm were reported. In another study a spray pyrolysis process using a ferrocene/acetonitrile solution was used. X-ray photo-electron spectroscopy studies revealed that the N content was indirectly proportional to the growth temperature, and TEM analysis showed that the bamboo compartment distance increased with an increase in growth temperature (Yadav et al.2009).
Albeit numerous articles have been published on the synthesis of N-CNTs, it is very difficult to compare the results obtained since different experimental conditions have been reported by the various authors.
Metal/N-CNT catalysts preparation
The primary aim in the preparation of a supported catalyst is to have a high surface area of a reduced metal deposited on a high surface area material. The nitrogen atom has an extra electron compared with carbon, and from an electronic point of view, therefore the surface of the tubes is expected to be more electronegative (Ayala et al. 2010). This results in N-CNTs possessing high surface nucleation sites, which allow the anchorage and high dispersion of the metal active phase on the support surface. In order to get more insight about the exact localization of the metal particles with respect to the N-CNT cavity, Chizari et al. (2010) used TEM tomography to study a Pd/N-CNT catalyst as presented in Fig. 4. According to the results, the palladium particles were exclusively restricted on the outer surface of the N-CNT which provides a highly effective metal surface contact for the reactants.
Molecular dynamics simulations were used to investigate the effect of ruthenium on the stability of N doped MWCNTs. Figure 5a depicts a model of nitrogen doped (substitutionally) on the CNT network. The system has been hydrogen-terminated to account for dangling bonds. Ruthenium was placed 2.048 Å (stable configuration) above the CNT surface and after simulations ruthenium was seen to be repelled from the surface to 4,044 and 3.147 Å for pristine CNT (Fig. 5b) and N-CNT (Fig. 5c), respectively. The configuration, Fig. 5c was comparably found to be the most stable. The results show that the nitrogen atoms act as donor-like atoms and not directly as binding sites for the deposited metal active phase.
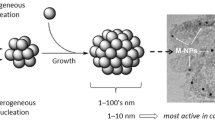
Currently, very few studies have been performed with N-CNTs as a support in catalysis as shown in Table 1. In order to use the N-CNTs effectively as a support for catalyst metal nanoparticles, they must be well dispersed on the support surface (Fig. 6). Supported metal catalysts are prepared by reducing a metal oxide and binding it onto a support material (Chakrabarty 1990). The dispersion of metal particles on CNTs by chemical means is based on conventional catalyst procedures such as by metal vaporisation or chemical reduction deposition from the solution phase (Chen et al. 2004). However, certain requirements are not always fulfilled when these methods are used. Below are some of the challenges experienced during catalyst preparation:
-
particle mean size is not well controlled;
-
wide particle size distribution of metals on CNTs;
-
particles are agglomerated and not uniformly dispersed (Zheng et al.2002);
-
cost issues associated with up-scaling.
The synthesis processes can also be time consuming since they include multiple steps such as long ageing, drying, and calcination of the samples. Hence, there is an interest in using alternative techniques such as microwave heating to achieve synthesis control.
Application of metal/N-CNTs in electro catalysis
Few applications of the use of N-CNTs as a support in fuel cells have been reported. Currently, the degradation of an electro-catalyst and its support are recognised as key contributors to the long-term degradation of fuel cells (Chen et al. 2009). Another major limitation to the commercialization of fuel cells is the high costs of their components. A study of catalysts for the oxygen reduction reaction (ORR) using nitrogen-containing carbon has propelled the use of N-CNT supported catalysts in fuel cells. N-CNTs have shown to be a highly active catalyst for the ORR in a proton exchange membrane (PEM) fuel cell environment. The conductivity of this material was slightly less than, but still comparable to materials used in fuel-cells (Matter et al.2007). In addition, Chen et al. (2009) evaluated the electrochemical activity of N-CNTs prepared with different catalysts using a rotating ring disc electrode (RRDE) voltammeter. The outcomes showed that the nitrogen content of N-CNTs is crucial for ORR. A high degree of N-CNT surface defects was observed, and it enhanced ORR activity by exposing more edge plane nitrogen groups such as pyridinic and pyrollic nitrogen, which could take part in the ORR through the lone pair electrons. In another study, the N-CNTs were used as a cathode material for the ORR in alkaline medium. Furthermore, the material proved to be more active as compared with commercially available Pt/C catalysts. The improved electrocatalytic activity showed two important features: a significant shift of the oxygen reduction and an increase in the oxygen reduction current, which is likely related to a higher average number of electrons transferred per oxygen molecule (Nagaiah et al. 2010). Similar results were obtained by Geng et al. (2010). The onset potential and current density of N-CNTs with nitrogen content of 7.7 wt% electrocatalyst reported in this work is equal to a platinum-based catalyst with a loading of 1.94 μg of platinum per cm2 at the electrochemical window tested in alkaline solution, showing that the N-CNTs have the potential to replace the costly Pt/C catalyst in alkaline fuel cells.
Lin et al. (2009) also investigated the effects of nitrogen incorporation into CNT structures on their electrochemical (EC) properties. Studies were carried out by correlating the nitrogen content of CNTs with EC performance. The results showed that nitrogen incorporation provided a simple pathway to modifying the electronic bonding structure. N-CNTs with optimal 3.5% N promoted a substitutional graphite-like defect structure, which is favourable for fast ET in electrochemistry. Albeit that there is a lack of a proposed mechanism for the ORR that includes a non-metallic active site, several studies have suggested how nitrogen can improve the ability of carbon to reduce oxygen. It is proposed that this is due to the ability of nitrogen to donate electrons to O2 (Matter et al.2006). N-CNTs also appear to produce a better electrode than Pt–C/glassy carbon (GC) since there is a substantial enhanced steady state diffusion current (~0.8 mA) for N-CNTs/GC with respect to Pt–C/GC electrodes (Fig. 7). Furthermore, N-CNT electrodes show stronger diffusion-limited currents and lower over potentials when compared with their nitrogen-free counterparts (Gong et al.2009).
CVs for the ORR at the Pt–C/GC (top) and N-CNT/GC (bottom) electrodes before (solid black curves) and after (dotted curves) a continuous potentiodynamic swept for ~100,000 cycles in an air saturated 0.1 M KOH solution at room temperature. Scan rate, 100 mV s−1. The wavelike bands over −1.0 to −0.5 V seen for the pristine Pt–C/GC electrode are attributable to hydrogen adsorption/desorption (Gong et al.2009)
Studies have related the importance of the nitrogen heteroatom in N-CNTs to their electrocatalytic activities for the ORR/fuel cells (Gong et al.2009). Given that nitrogen has an effect on the surface chemical activity and creates surface properties such as polarity, basicity, and heterogeneity, N-CNTs as catalyst supports are likely to increase the durability of the resultant catalyst (Chetty et al.2009). Chen et al. (2009) have shown that N-CNTs as support have advantages over pristine CNTs in Pt (platinum) catalytic activity. In their study, the electrochemical surface area (ESA) was used to characterise a proton exchange membrane fuel cell (PEMFC) catalyst. The degradation of the five catalysts is plotted in Fig. 8.
The durability of Pt/C was found to be much lower than that of Pt/CNTs. After 4,000 cycles, only 4.6% of the initial ESA of Pt/C remained while 11.2% of the initial ESA for Pt/CNTs was observed. It is also clear that Pt/N-CNTs have a higher stability than Pt/CNTs. Not only did the ESA increase, but accelerated durability tests and TEM results indicated that the stability of the Pt catalyst was increased by doping with N. Similar studies were done by Saha and co-workers (Saha et al.2009). They revealed that the Pt/N-CNT electrodes exhibited a greater electrochemical surface than the Pt/CNT electrodes as well as higher single-cell performance in a H2O2 fuel cell. In another study, Pt catalysts were loaded on three different supports: graphite, CNTs, and N-CNTs. In order to see the effect that incorporated nitrogen would have, the catalytic activity was evaluated by increasing the amount of nitrogen in the CNTs from 0 to 16.7%. The catalytic activity for methanol oxidation increased with an increase of the nitrogen content up to 10.5%. As shown in Table 2 the activity of an electrode with 10.5% nitrogen was higher than that obtained from the other N-CNTs, CNTs, and commercial Pt/C electrodes. The increasing order of stability of the various electrodes is Pt < Pt/Vulcan (E-TEK) < Pt/CNT < Pt/N-CNT1 < Pt/N-CNT2 < Pt/N-CNT3. It was also found that the metal particle distributions on the N-CNT support as well as the metal-support interactions are important parameters contributing to the activity of the catalyst. Thus, the higher activity of the Pt/N-CNT3 electrode with 10.5% nitrogen content may be attributed to the small particle size, higher dispersion of platinum and the nature of the CNT supports (Maiyalagan 2008).
Another advantage of N-CNTs as support for fuel cell application was illustrated by the study of PtRu nanoparticles dispersed on N-CNTs (Chetty et al.2009). The obtained catalyst was used to investigate the catalytic activity for the oxidation of methanol and compared with oxidised CNT and a commercial catalyst support. The cyclic voltammograms (refer to Fig. 9.) were recorded for the oxidation of methanol (1 mol dm−3) at a scan rate of 50 mV s−1 for PtRu/N-CNT, PtRu/O-CNT, PtRu/C E-TEK, and PtRu/Vulcan catalysts. An improvement in the activity was observed for the PtRu catalyst supported on the N-CNT support and was confirmed by the lower onset potential and significantly higher oxidation current [curve (i)] in the forward sweep. The oxidation peak observed in the reverse scan at around 0.4 V is associated with the oxidation of adsorbed intermediate species in the forward scan. The onset potential for PtRu/N-CNT was 0.21 V, while that for PtRu/O-CNT was 0.33 V. In principle, with respect to the methanol electrooxidation mechanism, the onset potential is related to the breaking of C–H bonds and the subsequent removal of the COads like intermediates by oxidation with OHads species supplied by Ru-OH sites. In the CO stripping study, PtRu supported on the N-CNTs showed the lowest onset potential confirming the easier oxidation and removal of the COads intermediates, which is reflected by the significantly higher methanol oxidation current.
Linear sweep voltammograms at 1 mV s−1 scan rate for the oxidation of 1 mol dm−3 methanol in 0.5 mol dm−3 H2SO4 at room temperature. Inset showing the chronoamperometric response recorded at 400 mV (vs. SCE). i PtRu/N-CNT, ii PtRu/O-CNT, iii E-TEK PtRu/C and iv PtRu/Vulcan catalysts (Chetty et al.2009)
Application of Metal/N-CNTs in heterogeneous catalysis
This high surface reactivity of N-CNTs is not only used in the electrocatalytic reactions but can also be used in other catalytic reactions. Ru catalysts were tested in the catalytic ammonia decomposition reaction. N-CNTs and conventional CNTs prepared under the same conditions were used as support for Ru. The activity of Ru improved significantly when supported on N-CNTs; the differences in the activity could not be accredited to the Ru particle size effect since the Ru particle sizes were similar in both cases (García-García et al. 2010). Chen et al. (2009) and García-García et al. (2010) also demonstrated that the decomposition reaction of ammonia was enhanced by the Ru catalyst on the N-CNTs. It was concluded that the catalytic activity of a Ru-based catalyst depends on the electron transfer ability and the dispersion of Ru nanoparticles. Recently, N-CNTs were used as a catalyst support for palladium in the liquid-phase hydrogenation of cinnamaldehyde. Palladium active phase supported on the N-CNTs showed C=C bond hydrogenation activity and selectivity higher than that found for the undoped CNTs. The specific areas of the two supports were similar (183 and 176 m2/g, respectively) (Amadou et al.2008). This is in agreement with the findings by Chen et al. (2009) that showed that the activity depends on the dispersion of the metal catalyst. The high dispersion of metal on the N-CNTs is attributed to the electronic interactions with the sp2 hybridized nitrogen with an unbonded electron pair, being able to complex the empty orbital of the metal ion as a ligand (Chizari et al. 2010). The electrocatalytic activity for ethanol oxidation, which has never been done before, was also investigated using Pt-NCNTs as catalyst. The report shows that Pt-NCNTs catalyst displayed a higher electrocatalytic activity than Pt-deposited on undoped CNTs (Zhu et al. 2010).
Conclusion
This article summarised the current literature on the use of N-CNTs as metal support. Currently there are few applications related to the use of N-CNTs as a catalyst support with fuel cell applications being significantly studied compared with other applications. N-CNTs are still in the early stages of development, but they have already positioned themselves as exceptional nanomaterials due to their surface and properties. In order for the N-CNTs to demonstrate their ability to act as nanoparticle supports, appropriate metal addition methods for nanoparticles must be exploited. The application of N-CNT metal catalyst is expected to expand in future.
References
Ajayan PM (1999) Nanotubes from carbon. Chem Rev 99:1787–1800
Amadou J, Chizari K, Houllé M, Janowska I, Ersen O, Bégin D, Pham-Huu C (2008) N-doped carbon nanotubes for liquid-phase CC bond hydrogenation. Catal Today 138:62–68
Antolini E (2009) Carbon supports for low-temperature fuel cell catalysts. Appl Catal B Environ 88:1–24
Ayala P, Arenal R, Rümmeli M, Rubio A, Pichler T (2010) The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 48:575–586
Bahome MC, Jewell LL, Padayachy K, Hildebrandt D, Glasser D, Datye AK, Coville NJ (2007) Fe-Ru small particle bimetallic catalysts supported on carbon nanotubes for use in Fischer–Tröpsch synthesis. Appl Catal 328:243–251
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes–the route toward applications. Science 297:787–792
Burch HJ (2006) Bioapplications of nitrogen-doped carbon nanotubes. In: Department of Physics (eds). University of Oxford
Chakrabarty DK (1990) Adsorption and catalysis by solids. Wiley Eastern Ltd., New Delhi
Chen W-X, Lee JY, Liu Z (2004) Preparation of Pt and PtRu nanoparticles supported on carbon nanotubes by microwave-assisted heating polyol process. Mater Lett 58:3166–3169
Chen C-C, Chen C-F, Chen C-M, Chuang F-T (2007) Modification of multi-walled carbon nanotubes by microwave digestion method as electrocatalyst supports for direct methanol fuel cell applications. Electrochem Commun 9:159–163
Chen Y, Wang J, Liu H, Li R, Sun X, Ye S, Knights S (2009) Enhanced stability of Pt electrocatalysts by nitrogen doping in CNTs for PEM fuel cells. Electrochem Commun 11:2071–2076
Chen J, Zhu ZH, Wang S, Ma Q, Rudolph V, Lu GQ (2010a) Effects of nitrogen doping on the structure of carbon nanotubes (CNTs) and activity of Ru/CNTs in ammonia decomposition. Chem Eng J 156:404–410
Chen Z, Higgins D, Chen Z (2010b) Electrocatalytic activity of nitrogen doped carbon nanotubes with different morphologies for oxygen reduction reaction. Electrochim Acta 55:4799–4804
Chen Z, Higgins D, Chen Z (2010c) Nitrogen doped carbon nanotubes and their impact on the oxygen reduction reaction in fuel cells. Carbon 48:3057-3065
Chetty R, Kundu S, Xia W, Bron M, Schuhmann W, Chirila V, Brandl W, Reinecke T, Muhler M (2009) PtRu nanoparticles supported on nitrogen-doped multiwalled carbon nanotubes as catalyst for methanol electrooxidation. Electrochim Acta 54:4208–4215
Chizari K, Janowska I, Houllé M, Florea I, Ersen O, Romero T, Bernhardt P, Ledoux MJ, Pham-Huu C (2010) Tuning of nitrogen-doped carbon nanotubes as catalyst support for liquid-phase reaction. Appl Catal A Gen 380:72–80
Chun K-Y, Lee HS, Lee CJ (2009) Nitrogen doping effects on the structure behavior and the field emission performance of double-walled carbon nanotubes. Carbon 47:169–177
Collins PG, Avouris P (2000) Nanotubes for electronics. Sci Am 283:62–69
Cruz-Silva E, Cullen DA, Gu L, Romo-Herrera JM, Muñoz-Sandoval E, López-Urías F, Sumpter BG, Meunier V, Charlier J-C, Smith DJ, Terrones H, Terrones M (2008) Heterodoped nanotubes: theory, synthesis, and characterization of phosphorus nitrogen doped multiwalled carbon nanotubes. ACS Nano 2:441–448
Dai H, Hafner JH, Rinzler AG, Colbert DT, Smalley RE (1996) Nanotubes as nanoprobes in scanning probe microscopy. Nature 384:147–150
Dresselhaus MS, Dresselhaus G, Eklund PC (1996) Science of fullerenes and carbon nanotubes. Academic press, New York
Dresselhaus MS, Dresselhaus G, Avouris P (2001) Carbon nanotubes: synthesis, structure, properties, and applications. Springer, Berlin
Du HY, Wang CH, Hsu HC, Chang ST, Chen US, Yen SC, Chen LC, Shih HC, Chen KH (2008) Controlled platinum nanoparticles uniformly dispersed on nitrogen-doped carbon nanotubes for methanol oxidation. Diam Relat Mater 17:535–541
Ebbesen TW, Lezec HJ, Hiura H, Bennett JW, Ghaemi HF, Thio T (1996) Electrical conductivity of individual carbon nanotubes. Nature 382:54–56
Fan Y-Y, Kaufmann A, Mukasyan A, Varma A (2006) Single- and multi-wall carbon nanotubes produced using the floating catalyst method: synthesis, purification and hydrogen up-take. Carbon 44:2160–2170
Gao G-Y, Guo D-J, Li H-L (2006) Electrocatalytic oxidation of formaldehyde on palladium nanoparticles supported on multi-walled carbon nanotubes. J Power Sources 162:1094–1098
Garcia J, Gomes HT, Serp P, Kalck P, Figueiredo JL, Faria JL (2006) Carbon nanotube supported ruthenium catalysts for the treatment of high strength wastewater with aniline using wet air oxidation. Carbon 44:2384–2391
García-García FR, Álvarez-Rodríguez J, Rodríguez-Ramos I, Guerrero-Ruiz A (2010) The use of carbon nanotubes with and without nitrogen doping as support for ruthenium catalysts in the ammonia decomposition reaction. Carbon 48:267–276
Geng D, Liu H, Chen Y, Li R, Sun X, Ye S, Knights S (2010) Non-noble metal oxygen reduction electrocatalysts based on carbon nanotubes with controlled nitrogen contents. J Power Sources 196:1795–1801
Ghosh P, Soga T, Ghosh K, Afre RA, Jimbo T, Ando Y (2008a) Vertically aligned N-doped carbon nanotubes by spray pyrolysis of turpentine oil and pyridine derivative with dissolved ferrocene. J Non Cryst Solids 354:4101–4106
Ghosh P, Tanemura M, Soga T, Zamri M, Jimbo T (2008b) Field emission property of N-doped aligned carbon nanotubes grown by pyrolysis of monoethanolamine. Solid State Commun 147:15–19
Ghosh K, Kumar M, Maruyama T, Ando Y (2010) Tailoring the field emission property of nitrogen-doped carbon nanotubes by controlling the graphitic/pyridinic substitution. Carbon 48:191–200
Gong K, Du F, Xia Z, Durstock M, Dai L (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323:760–764
Guczi L, Stefler G, Geszti O, Koppány Z, Kónya Z, Molnár É, Urbán M, Kiricsi I (2006) CO hydrogenation over cobalt and iron catalysts supported over multiwall carbon nanotubes: effect of preparation. J Catal 244:24–32
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Jiang S, Zhu L, Ma Y, Wang X, Liu J, Zhu J, Fan Y, Zou Z, Hu Z (2010) Direct immobilization of Pt–Ru alloy nanoparticles on nitrogen-doped carbon nanotubes with superior electrocatalytic performance. J Power Sources 195:7578–7582
Koós AA, Dowling M, Jurkschat K, Crossley A, Grobert N (2009) Effect of the experimental parameters on the structure of nitrogen-doped carbon nanotubes produced by aerosol chemical vapour deposition. Carbon 47:30–37
Ledoux M-J, Pham-Huu C (2005) Carbon nanostructures with macroscopic shaping for catalytic applications. Catal Today 102–103:2–14
Li J, Zhang Y (2005) A simple purification for single-walled carbon nanotubes. Phys E Low Dimensional Syst Nanostruct 28:309–312
Li H, Zhao N, He C, Shi C, Du X, Li J, Cui Q (2008) Fabrication of short and straight carbon nanotubes by chemical vapor deposition. Mater Sci Eng A 476:230–233
Li H, Liu H, Jong Z, Qu W, Geng D, Sun X, Wang H (2011) Nitrogen-doped carbon nanotubes with high activity for oxygen reduction in alkaline media. Int J Hydrogen Energy 36(3):2258–2265
Liang Y, Zhang H, Yi B, Zhang Z, Tan Z (2005) Preparation and characterization of multi-walled carbon nanotubes supported PtRu catalysts for proton exchange membrane fuel cells. Carbon 43:3144–3152
Lin YG, Hsu YK, Wu CT, Chen SY, Chen KH, Chen LC (2009) Effects of nitrogen-doping on the microstructure, bonding and electrochemical activity of carbon nanotubes. Diam Relat Mater 18:433–437
Liu J, Webster S, Carroll DL (2005) Temperature and flow rate of NH3 effects on nitrogen content and doping environments of carbon nanotubes grown by injection CVD method. J Phys Chem B 109:15769–15774
Maiyalagan T (2008) Synthesis and electro-catalytic activity of methanol oxidation on nitrogen containing carbon nanotubes supported Pt electrodes. Appl Catal B Environ 80:286–295
Maldonado S, Morin S, Stevenson KJ (2006) Structure, composition, and chemical reactivity of carbon nanotubes by selective nitrogen doping. Carbon 44:1429–1437
Martínez-Méndez S, Henríquez Y, Domínguez O, D’Ornelas L, Krentzien H (2006) Catalytic properties of silica supported titanium, vanadium and niobium oxide nanoparticles towards the oxidation of saturated and unsaturated hydrocarbons. J Mol Catal A Chem 252:226–234
Matlhoko L, Pillai SK, Moodley M, Augustyn WG, Ray SS (2009) A comparison of purification procedures for multi-walled carbon nanotubes produced by chemical vapour deposition. J Nanosci Nanotechnol 9:5431–5435
Matter PH, Wang E, Ozkan US (2006) Preparation of nanostructured nitrogen-containing carbon catalysts for the oxygen reduction reaction from SiO2- and MgO-supported metal particles. J Catal 243:395–403
Matter PH, Wang E, Arias M, Biddinger EJ, Ozkan US (2007) Oxygen reduction reaction activity and surface properties of nanostructured nitrogen-containing carbon. J Mol Catal A Chem 264:73–81
Mhlanga S (2009) Carbon nanotubes with CaCO3 as support for catalysts. In: Chemistry Department (eds). University of the Witwatersrand, Gauteng
Montoro LA, Rosolen JM (2006) A multi-step treatment to effective purification of single-walled carbon nanotubes. Carbon 44:3293–3301
Nagaiah TC, Kundu S, Bron M, Muhler M, Schuhmann W (2010) Nitrogen-doped carbon nanotubes as a cathode catalyst for the oxygen reduction reaction in alkaline medium. Electrochem Commun 12:338–341
Nieto-Márquez A, Lazo JC, Romero A, Valverde JL (2008) Growth of nitrogen-doped filamentous and spherical carbon over unsupported and Y zeolite supported nickel and cobalt catalysts. Chem Eng J 144:518–530
Nxumalo EN, Letsoalo PJ, Cele LM, Coville NJ (2010) The influence of nitrogen sources on nitrogen doped multi-walled carbon nanotubes. J Organomet Chem 695:2596–2602
Osváth Z, Osváth Z, Koos AA, Vértesy Z, Horváth ZE, Biró LP (2009) Scanning tunneling microscopy and spectroscopy of nitrogen doped multi-walled carbon nanotubes produced by the pyrolysis of ferrocene and benzylamine. J Nanosci Nanotechnol 9:6139–6143
Panchakarla LS, Govindaraj A, Rao CNR (2010) Boron- and nitrogen-doped carbon nanotubes and graphene. Inorganica Chim Acta (in press, corrected proof)
Reddy BM, Rao KN, Reddy GK, Bharali P (2006) Characterization and catalytic activity of V2O5/Al2O3-TiO2 for selective oxidation of 4-methylanisole. J Mol Catal A Chem 253:44–51
Reyes-Reyes M, Grobert N, Kamalakaran R, Seeger T, Golberg D, Rühle M, Bando Y, Terrones H, Terrones M (2004) Efficient encapsulation of gaseous nitrogen inside carbon nanotubes with bamboo-like structure using aerosol thermolysis. Chem Phys Lett 396:167–173
Saha MS, Kundu A (2010) Functionalizing carbon nanotubes for proton exchange membrane fuel cells electrode. J Power Sources 195:6255–6261
Saha MS, Li R, Sun X, Ye S (2009) 3-D composite electrodes for high performance PEM fuel cells composed of Pt supported on nitrogen-doped carbon nanotubes grown on carbon paper. Electrochem Commun 11:438–441
Shao Y, Sui J, Yin G, Gao Y (2008) Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Appl Catal B Environ 79:89–99
Stephan O, Ajayan PM, Colliex C, Redlich P, Lambert JM, Bernier P, Lefin P (1994) Doping graphitic and carbon nanotube structures with boron and nitrogen. Science 266:1683–1685
Sumpter BG, Meunier V, Romo-Herrera JM, Cruz-Silva E, Cullen DA, Terrones H, Smith DJ, Terrones M (2007) Nitrogen-mediated carbon nanotube growth: diameter reduction, metallicity, bundle dispersability, and bamboo-like structure formation. ACS Nano 1:369–375
Tao XY, Zhang XB, Sun FY, Cheng JP, Liu F, Luo ZQ (2007) Large-scale CVD synthesis of nitrogen-doped multi-walled carbon nanotubes with controllable nitrogen content on a CoxMg1-xMoO4 catalyst. Diam Relat Mater 16:425–430
Thostenson ET, Ren Z, Chou T-W (2001) Advances in the science and technology of carbon nanotubes and their composites: a review. Compos Sci Technol 61:1899–1912
Treacy MMJ, Ebbesen TW, Gibson JM (1996) Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 381:678–680
Van Dommele S, Romero-Izquirdo A, Brydson R, de Jong KP, Bitter JH (2008) Tuning nitrogen functionalities in catalytically grown nitrogen-containing carbon nanotubes. Carbon 46:138–148
Villalpando-Paez F, Zamudio A, Elias AL, Son H, Barros EB, Chou SG, Kim YA, Muramatsu H, Hayashi T, Kong J, Terrones H, Dresselhaus G, Endo M, Terrones M, Dresselhaus MS (2006) Synthesis and characterization of long strands of nitrogen-doped single-walled carbon nanotubes. Chem Phys Lett 424:345–352
Yadav R, Dobal P, Shripathi T, Katiyar R, Srivastava O (2009) Effect of growth temperature on bamboo-shaped carbon–nitrogen (C–N) nanotubes synthesized using ferrocene acetonitrile precursor. Nanoscale Res Lett 4:197–203
Yu M-F, Lourie O, Dyer MJ, Moloni K, Kelly TF, Ruoff RS (2000) Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 287:637–640
Zheng B, Li Y, Liu J (2002) CVD synthesis and purification of single-walled carbon nanotubes on aerogel-supported catalyst. Appl Phys A 74:345–348
Zhu Z, Wang J, Munir A, Zhou HS (2010) Electrocatalytic activity of Pt nanoparticles on bamboo shaped carbon nanotubes for ethanol oxidation. Electrochim Acta 55:8517–8520
Acknowledgments
The authors would like to thank the University of the Witwatersrand, CSIR and DST for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mabena, L.F., Sinha Ray, S., Mhlanga, S.D. et al. Nitrogen-doped carbon nanotubes as a metal catalyst support. Appl Nanosci 1, 67–77 (2011). https://doi.org/10.1007/s13204-011-0013-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-011-0013-4