Abstract
The hetero-system CuFeO2/ZnO was successfully used in the Cu2+ reduction under visible light irradiation; it was conducted with varying some important factors such as the initial Cu2+ concentration, mass ratio CuFeO2/ZnO and light flux. The Cu2+ reduction obeys to a first-order kinetic with a half-life of 60 min. The regression in the reduction rate over time is due to the competitive hydrogen evolution. Indeed, the electrons are transferred to water via Cu2+ clusters. Also as application, the H2 evolution is successfully achieved with an evolution rate of 3.42 µmol (g catalyst)−1 min−1 and a quantum yield of 0.5%. The hydrogen is facilitated through generated bifunctional hetero-system because of the low H2 overvoltage on copper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mankind is living in an environment with toxic compounds including heavy metals (Tack et al. 2017; Boulaiche et al. 2019). They are fatal for the human health, and the World Health Organization (WHO) has drastically reduced their concentrations in water (Liang et al. 2015). Unlike organic molecules, metals are persistent and non-biodegradable; they appear in water through industrial activities and accumulate in living organisms. Therefore, their elimination is a concern worldwide and the effluents must be treated at the source before being released in the aquatic medium.
Conventionally, the treatment includes physical methods such as the ions exchange, reverse osmosis, adsorption and electro-reduction (Abbas et al. 2014; Azoudj et al. 2018; Quibén et al. 2018; Yeddou Mezenner et al. 2017). However, such methods remain high cost and some of them are inefficient at low concentrations. Therefore, an effective treatment is required and the photocatalysis provides an efficient method for the water depollution (Bagtache et al. 2018). The technique is based on the excitation of a semiconductor (SC) by energetic photons (hν > Eg), Eg being the optical gap. The generated electron/hole (e−/h+) pairs are used to degrade organic pollutant via radicals O2· and ·OH and/or to reduce water to hydrogen (Bagtache et al. 2016) and metals ions into less harmful forms (Cherifi et al. 2019; Saien and Azizi 2015). The subject is pertinent to the environmental protection, and one of the challenges in photo-electrochemical (PEC) devices is the reduction of the material cost. In this respect, the delafossites Cu+M3+O2, where M denotes 3d metal, have attracted a great deal of attention as photocatalysts (Bellal et al. 2009), and among the candidates, CuFeO2 absorbs over the whole solar spectrum and is water resistant (Omeiri et al. 2008; Bassaid et al. 2009). In addition, Fe is attractive with regard to toxicity concern. On the other hand, copper is an oligo-element but becomes toxic above a threshold concentration of 1 ppm.
The photoactivity is a surface phenomenon and depends on the morphology of the powder. Large active surfaces are needed for low polaron oxides like CuFeO2 because of the low diffusion length of the minority carriers (Bassaid et al. 2009). On the other hand, the photocatalytic performance can be significantly improved on hetero-systems because they produce an optimal band bending at the interface semiconductor/electrolyte (Derbal et al. 2008; Qin et al. 2019). Hence, the visible light is not converted with ZnO and affords a poor PEC activity but ZnO can be used as Electron Bridge between CuFeO2 and Cu2+ and favors the separation of (e−/h+) pairs. Moreover, ZnO has attracted a great attention as window material owing to its light transparency for the visible light.
Herein, we prepared for the first time the hetero-system CuFeO2/ZnO by nitrate route. We report for CuFeO2 and ZnO the flat band potential (Efb), the chemical stability, the optical gap and the position of their conduction bands. Theses characterizations make it possible to draw the energetic diagram of CuFeO2/ZnO which was used for the Cu2+ reduction. Many parameters are studied like the initial Cu2+ concentration, the mass ratio of CuFeO2/ZnO, stirring speed and light intensity. Also the tri-functional system CuFeO2/ZnO/Cu was evaluated for the hydrogen production under visible light.
Experimental
The preparation procedure of CuFeO2 was reported elsewhere (Omeiri et al. 2008). ZnO is prepared by dehydration and denitrification of Zn(NO3)2.6H2O (Panreac, 98%) at 200 °C; then, the powder is heat-treated at 400 °C. The phases were identified by X-ray diffraction (XRD) using Cu Kα radiation (λ = 0.15406 nm).
The optical gap was determined by using a Jasco V-650 spectrophotometer attached to an integrating sphere. The data were collected in the range (190–900 nm), and BaSO4 was used as standard.
CuFeO2 pellets pressed under a pressure of 5 kbar and sintered at 950 °C exhibit a density of ~ 80%. A copper wire was attached to the back pellet with silver cement to insure an ohmic contact. The pellet was enrobed in a glass tube with epoxy resin leaving a cross section of 0.5 cm2. The electrode was polished with alumina paste and thoroughly washed with distilled water. The electrochemical study was conducted in a standard cell; the emergency electrode consists of a platinum flag sealed in a soft glass and cleaned in HNO3 (65%). The potential of the working electrode was monitored by a PGZ 301 potentiostat (radiometer analytical); it was given with respect to a saturated calomel electrode (SCE) and uncorrected for the junction potentials. Because of the p-type behavior of CuFeO2, the potential was ramped from + 1 V toward cathodic direction at a scan rate of 5 mV s−1 in CuSO4 electrolyte.
The photocatalytic tests were done in a double-walled reactor (500 mL total capacity) at atmospheric pressure; the temperature was regulated at 25 °C. Typically, 200 mg of the catalyst was dispersed in 200 mL of Cu2+ solution, prepared from CuSO4 (Merck). The amount of adsorbed Cu2+ was evaluated by titration after keeping the powder in solution overnight. A tungsten lamp (200 W, Sonelec, Algeria) with a maximum emission at 620 nm was used as light source. The aliquots were removed at regular time intervals, and the precipitate was separated by centrifugation (5000 rpm, 15 min). The aliquots were withdrawn at periodic time for the Cu2+ titration. After vigorous centrifugation, the analysis was carried out by inductively coupled plasma (ICP, Varian Vista-Pro-CCD and Simultaneous ICP-OES). The concentration was determined by interpolation from a calibration plot.
Evolved hydrogen was evaluated volumetrically; hydrogen rises to the top of the vial and displaces the solution up the graduate tube. Distilled water (~ 0.8 Ω cm) was used in all experiments.
Results and discussion
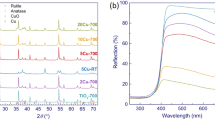
The co-precipitation has succeeded in the synthesis of homogeneous delafossites; the technique consumes less energy and time and produces submicron-sized grains with increased active surface areas (Omeiri et al. 2008). The heat treatment gave a dark phase and CuFeO2 nano-crystallites (~ 40 nm) were obtained from the full width t half maxim (FWHM). All XRD peaks are assigned to CuFeO2 (Fig. 1) and indexed in a hexagonal structure (SG: R\(\overline{3}\)m), JCPDS card N° (39-0246) with the cell constants: a = 303.3 nm, c = 1713.6 nm. The structure defects affect the electrical properties, and the delafossites exhibit p-type SCs because of the oxygen incorporation in the layered crystal lattice (Abdi et al. 2018).
ZnO obtained from nitrate decomposition crystallizes in the wurtzite structure with a hexagonal symmetry (SG: P 6/3mc); the lattice constant, a = 0.324552 nm, c = 0.520640 nm, agree with the JCPDS N° 36-1451 as shown in inset (Fig. 1). The gap (Eg) of CuFeO2 (Fig. 2) is determined from the diffuse reflectance spectrum by using the Pankov relation (Kabouche et al. 2017)
where m is equal to 0.5 and 2 for indirect and direct allowed transition, respectively. The oxide is a narrow band gap semiconductor with Eg value of 1.32 eV, close to the optimal value for the solar energy conversion and assigned to d–d transition of Cu+ linearly coordinated. The potential of photoelectrons in the conduction band (− 0.92 VSCE) is negative enough to reduce Cu2+ into elemental state. ZnO has band gap energy of Eg = 3.2 eV (Helaïli et al. 2010) and is active under visible light, but its spectral photoresponse is extended to visible region in the hetero-system.
The photocatalysis needs not only a gap of ~ 1.5 eV but also a matching of electronic bands with the redox couples. In this respect, the energy electronic bands are determined from the capacitance measurements. The dark current is low, and the capacity of the space charge region gives an accurate value of the flat band potential (Efb). The value of − 0.71 V obtained for ZnO is provided from the intercept of the potential-axis at C−2 = 0 of the capacitance given by the relation (Fig. 3b):
The symbols have their usual significations. The positive slope of ZnO is characteristic of n-type conduction (Fig. 3b). On the contrary, CuFeO2 shows p-type behavior with Efb value of + 0.36 eV (Fig. 3a).
As noticed above, the photoactivity is dependent on the conditions of preparation and the improved performance is due to increased active surface area of ZnO on which the Cu2+ adsorption occurs. The adsorption on ZnO is pH dependent and is pronounced below pzc (= 7.23) where the Cu2+ ions are attracted by ZnO surface, negatively charged; the equilibrium dark is reached after ~ 2 h. However, to ensure a total adsorption, the suspension was kept overnight in the dark.
The Cu2+ reduction is non-spontaneous, and the light is used for its reduction. In addition, the free potential of p-CuFeO2 is more negative than the potential Efb and there is no need to apply an additional voltage to drive the uphill reaction as it occurs under cathodic polarization. The energy diagram (Fig. 4), drawn from the optical and electrochemical characterizations at pH ~ 7, shows that Cu2+ has a potential above ZnO-CB and can be reduced spontaneously upon illumination on the hetero-system p-CuFeO2/n-ZnO.
CuFeO2 works as electrons reservoir; its illumination excites electrons to the conduction band which are injected into ZnO-CB acting as electro-catalytic centers for the charge transfer to adsorbed Cu2+. The reduction no longer holds if the potential of the couple Cu2+/Cu is located outside the forbidden band of ZnO.
The reduction is optimized with respect to the catalyst dose, Cu2+concentration and light intensity. The concentration of copper in industrial effluents can reach 10 ppm or more. Therefore, it is of interest to study the photoactivity as a function of Cu2+ concentration (Co). As expected, clearly shows that the Cu2+ reduction decreases with augmenting Co (Fig. 5). At low concentrations, the number of the photocatalytic sites is greater than Cu2+ ions and the photoactivity increases linearly with raising Co until occupation of all sites. The photons flux was maintained constant (6.96 × 1018 photons s−1) and increasing Co decelerates the photoactivity because of the saturation of photo-electrochemical sites of ZnO. Water reduction on Cu clusters also accounts for the regression of the activity (see below).
The influence of the mass ratio (CuFeO2/ZnO) on the photoactivity is illustrated in Fig. 6. As expected, the activity increases with augmenting the mass of the sensitizer CuFeO2 which and the best performance is obtained for a ratio of 1, this is simply due to the increasing number of photo-electrochemical sites with a higher receptive surface for incident photons. It slightly decreases when further CuFeO2 is added. The light scattering also accounts for the decreased activity.
The amount of deposited copper depends on the light flux (Fig. 7). A linear behavior is observed at small intensities, and the plot deviates from linearity above 9 mW cm−2. The plateau region at high intensities indicates that the number of incident photons exceeds that of catalytic sites and the reduction becomes kinetically governed by the electrons transport in CuFeO2. Indeed, the rate of the overall reaction is determined by the electron flow within CuFeO2-CB whose width does not exceed ~ 2 eV. Therefore, the electrons with a low mobility (~ 10–6 cm2 V−1 s−1) and a small diffusion length are rate determining step (Yong and Ling 2014; Abbasi and Arvand 2018). So it is interesting to work under direct sunlight where the diffuse nature of the sun allows low investment and where solar concentrators are not needed; the Cu2+ reduction follows a first-order kinetic with a half-life of 40 min (Fig. 8). The Cu-photodeposition occurs after 160 min and the quantum yield, determined by the number of deposited Cu divided by the number of photons (P = 6.96 × 1018 photons s−1) is found to be 0.36%
Light catalysis results in the deposition of ultra-fine Cu particles onto ZnO and over time; the Cu2+ reduction slows down because of the competitive water discharge. Indeed, the ZnO catalyst modified by Cu photodeposition gives heterogeneous surface and offers active sites for the water reduction into hydrogen where the electrons are channeled toward Cu sites. It is tempting to postulate the in situ photodeposition of Cu over ZnO leads to a better ohmic contact CuFeO2/ZnO/Cu and Cu deposits are grown by electrocrystallization.
The interfacial charge transfer occurs easily through the generated hetero-junction because of the low H2 over potential on Cu-sites. This can be easily understood in terms of energy levels of CB–CuFeO2, Cu2+ and H2O. The potential of the couple H2O/H2 can be positioned anywhere and the value at which the Jd(E) curve intercepts the potential-axis depends on the oxygen partial pressure. In air-equilibrated suspension, a potential − 0.70 V is obtained by extrapolating a tangent line over the slope and prolonging it to the zero current (Fig. not shown). The volume of evolved gas remains weak until ~ 40 min, and this means that at the beginning, the photoelectrons are consumed to reduce Cu2+. Once, the Cu-clusters are formed they trigger the H2 liberation. The quantum yield η (H2) of H2-formation for a maximal volume V(H2) (= 2.64 mL) at saturation time t (= 140 min):
where N is the Avogadro number, λ the average wave lamp of the lamp spectrum, Vm the molar volume, P the flux intensity, and S the surface area of the reactor. A value of 0.5% was obtained under optimized conditions (see SM 1). Work is continuing and preliminary tests on spinel Ni2+ deposition have been conclusive and the results will be communicated very soon.
Conclusion
The choice of CuFeO2 for the Cu2+ deposition is motivated by the light absorption in the visible IR regions, low cost and chemical stability over the whole pH range and the environmental friendless characteristic. However, its conduction band has a high energy for electron transfer and ZnO was used as electron bridge. The enhanced activity is attributed to the increased active surface area of ZnO on which Cu2+ is adsorbed.
Photocatalysis results in the deposition of ultra-fine Cu particles onto ZnO, providing an ohmic contact where the interfacial charge transfer occurs with small activation energy.
The hydrogen evolution is obtained after Cu-photodeposition dispersed in fine clusters over ZnO. The hetero-junction CuFeO2/ZnO/Cu became photocatalytically active for the hydrogen evolution which takes place competitively with Cu2+ reduction and therefore seems to be the main reason in the regression of the Cu2+ conversion as over illumination time.
References
Abbas M, Kaddour S, Trari M (2014) Kinetic and equilibrium studies of cobalt adsorption on apricot stone activated carbon. J Ind Eng Chem 20:745–751
Abbasi L, Arvand M (2018) Engineering hierarchical ultrathin CuCo2O4 nanosheets array on Ni foam by rapid electrodeposition method toward high-performance binder-free supercapacitors. Appl Surf Sci 445:272–280
Abdi A, Bagtache R, Trari M (2018) Physical and photo-electrochemical properties of oxygen rich delafossite CuYO225. J Solid State Electrochem. https://doi.org/10.1007/s10008-018-4031
Azoudj Y, Merzougui Z, Rekhila G, Trari M (2018) The adsorption of HCrO4− on activated carbon of date pits and its photoreduction on the hetero-system ZnCo2O4/TiO2. Appl Water Sci 8:114–121
Bagtache R, Abdmeziem K, Reghila G, Trari M (2016) Synthesis and semiconducting properties of Na2MnPO4F. Application to degradation of Rhodamine B under UV-light. Mater Sci Semicond Process 51:1–7
Bagtache R, Abdmeziem K, Dib K, Trari M (2018) Synthesis and photoelectrochemical characterization of KZn2(HPO4)PO4: application to rhodamine B photodegradation under solar light. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-018-1883-8
Bassaid S, Chaib M, Omeiri S, Bouguelia A, Trari M (2009) Photocatalytic reduction of cadmium over CuFeO2 synthesized by sol–gel. J Photochem Photobiol A Chem 201:62–68
Bellal B, Saadi S, Koriche N, Bouguelia A, Trari M (2009) Physical properties of the delafossite LaCuO2. J Phys Chem Solids 70:1132–1136
Boulaiche W, Hamdi B, Trari M (2019) Removal of heavy metals by chitin: equilibrium, kinetic and thermodynamic studies. Appl Water Sci 9:39–48
Cherifi K, Rekhila G, Omeiri S, Bessekhouad Y, Trari M (2019) Physical and photoelectrochemical properties of the spinel ZnCr2O4 prepared by sol gel: application to Orange II degradation under solar light. J Photochem Photobiol A 368:290–295
Derbal A, Omeiri S, Bouguelia A, Trari M (2008) Characterization of new heterosystem CuFeO2/SnO2 application to visible-light induced hydrogen evolution. Int J Hydrogen Energy 33:4274–4282
Helaïli N, Bessekhouad Y, Bouguelia A, Trari M (2010) p-Cu2O/n-ZnO hetero junction applied to visible light Orange II degradation. Sol Energy 84:1187–1192
Kabouche S, Bellal B, Louafi Y, Trari M (2017) Synthesis and semiconducting properties of tin (II) sulfide: application to photocatalytic degradation of Rhodamine B under sun light. Mater Chem Phys 195:229–235
Liang R, Jing F, Shen L, Qin N, Wu L (2015) MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J Hazard Mater 287:364–372
Omeiri S, Gabès Y, Bouguelia A, Trari M (2008) Photoelectrochemical characterization of the delafossite CuFeO2: application to removal of divalent metals ions. J Electroanal Chem 614:31–40
Qin C, Wang Y, Gong Y, Zhang Z, Cao J (2019) CuO-ZnO hetero-junctions decorated graphitic carbon nitride hybrid nanocomposite: hydrothermal synthesis and ethanol gas sensing application. J Alloy Compd 770:972–980
Quibén JC, Abdelwahab A, Cadenas MP, Torres SM, Cadenas AFP (2018) Carbon–iron electro-catalysts for CO2 reduction. The role of the iron particle size. J CO2 Util 24:240–249
Saien J, Azizi A (2015) Simultaneous photocatalytic treatment of Cr(VI), Ni(II) and SDBS in aqueous solutions: evaluation of removal efficiency and energy consumption. Process Saf Environ Prot 95:114–125
Tack FMG, Rinklebe J, Sik Y, Tsang DCW (2017) International conference on heavy metals in the environment (ICHMET). Chemosphere 185:94–95
Yeddou Mezenner N, Lagha H, Kais H, Trari M (2017) Biosorption of diazinon by a pre-treated alimentary industrial waste: equilibrium and kinetic modeling. Appl Water Sci 7:4067–4076
Yi LX, Yong Z, Ling Y (2014) Effect of various precipitants on activity and thermal stability of CuFe2O4 water-gas shift catalysts. J Fuel Chem Technol 42:1087–1092
Acknowledgements
The financial support of the work was provided by the Faculty of Chemistry. The authors are grateful to Dr. R. Laib for his technical assistance in the X-ray diffraction.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors attest that there are not conflict of interest financial, personal or other relationships with other people, laboratories or organizations worldwide.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bagtache, R., Missaoui, A., Korib, A. et al. The Cu2+ reduction and H2 evolution on the hetero-system CuFeO2/ZnO under visible light. Appl Water Sci 10, 134 (2020). https://doi.org/10.1007/s13201-020-01220-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01220-4