Abstract
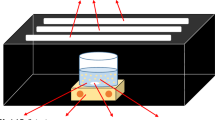
This work reports for the first time the study of the physical and photoelectrochemical properties of KZn2 (HPO4) PO4 nanopowder, prepared by hydrothermal route at 453 K. The as-synthesized compound crystallizes in a triclinic system (space group: P\(\bar{1}\)) with the lattice constants: a = 5.2060(2) Å, b = 8.8524(3) Å, c = 9.4039(3) Å, α = 96.9810(10)°, β = 101.7410(10)°, γ = 106.8130(10)° and a crystallite size of ~ 90 nm. The observed direct optical transition of 2.88 eV is due to O2−: 2p → Zn2+: 4s charge transfer; a further indirect transition at 5.80 eV is noticed. The electrical conductivity follows an exponential type law: σ = σo exp (− 0.08 eV/kT) in the temperature range (385–500 K), with an electronic hopping through mixed valences, while the negative thermo-power indicates n-type behavior. The capacitance measurement at pH ~ 7 gives a flat band potential of − 0.13 VSCE for the conduction band, which derives from Zn2+: 4s orbital and a donor density of 3.18 × 1019 cm−3. The electrochemical impedance spectroscopy shows the predominance of the faradic charge transfer. In order to support the photoelectrochemical results, the photocatalytic degradation of rhodamine B (RhB) in the presence of KZn2(HPO4)PO4, under solar light, is conducted. The results indicate 40% conversion after 300 min of irradiation time. A degradation mechanism is proposed to explain the mineralization. The photocatalytic RhB degradation obeys to a second-order kinetic model with a rate constant of 1.48 × 10−3 mol−1 L min−1.









Similar content being viewed by others
References
Aazam ES, Mohamed RM (2013) Environmental remediation of direct blue dyes solutions by photocatalytic oxidation with cuprous oxide. J Alloys Compd 577:550–555
Abid MF, Zablouk MA, Abid-Alameer AM (2012) Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration, Iranian. J Environ Health Sci Eng 9:9–17
Abo-Farha SA (2010) Comparative study of oxidation of some azo dyes by different advanced oxidation processes: fenton, fenton-like, photo-fenton and photo-fenton-like. J Am Sci 6:128–142
Al-Kahtani AA (2017) Photocatalytic degradation of rhodamine B dye in wastewater using gelatin/cus/PVA nanocomposites under solar light irradiation. J Biomater Nanobiotechnol 8:66–82
Averbuch-Pouchot MT (1979) Structure du monophosphate acide de potassium-zinc: KZn2H(PO4)2. Acta Cryst B 35:1452–1454
Bagtache R, Abdmeziem K, Rekhila G, Trari M (2016) Synthesis and semiconducting properties of Na2MnPO4F. Application to degradation of Rhodamine B under UV-light. Mater Sci Semicond Process 51:1–7
Byrappa K, Subramani AK, Ananda S, Lokanatha Rai KM, Dinesh R, Yoshimura M (2006) Photocatalytic degradation of rhodamine B dye using hydrothermally synthesized ZnO. Bull Mater Sci 29:433–438
Chavez AV, Neno TM, Hannooman L, Harrison WTA (1999) Tetrahedral-network organo-zincophosphates: syntheses and structures of (N2C6H14)·Zn(HPO4)2·H2O·H3N(CH2)3NH3·Zn2(HPO4)3, and (N2C6H14)·Zn3(HPO4)4. J Solid State Chem 147:584–591
Chen Y, He L, Li J, Cheng X, Lu H (2016) An inexact bi-level simulation-optimization model for conjunctive regional renewable energy planning and air pollution control for electric power generation systems. Appl Energy 183:969–983
Chidambaram D, Neeraj S, Natarajan S, Rao CNR (1999) Open-framework zinc phosphates synthesized in the presence of structure-directing organic amines. J Solid State Chem 147:154–169
Cotto-Maldonado M (2013) Photocatalytic degradation of rhodamine-B under UV–visible light irradiation using different nanostructured catalysts. Am Chem Sci J 3:178–202
Echavarrıá A, Masseron AS, Paillaud J-L, Gramlich V, Saldarriaga C (2003) Synthesis and characterisation of the layered zinc phosphate KZn2(PO4)(HPO4). Inorg Chim Acta 343:51–55
el Alouani M, Alehyen S, EL Achouri M, Taibi M (2018) Removal of cationic dye-methylene Blue- from aqueous solution by adsorption on fly ash-based geopolymer. J Mater Environ Sci 9:32–46
Fernández LT, Khainakova OA, Espina A, Amghouz Z, Khainakov SA, Alfonso BF, Blanco JA, García JR, Granda SG (2015) Hydrothermal synthesis and characterization of a two-dimensional piperazinium cobalt–zinc phosphate via a metastable one-dimensional phase. J Solid State Chem 225:340–346
Hachoumi I, El-Ouahabi I, Slimani R, Cagnon B, El Haddad M, El Antri S, Lazar S (2017) Adsorption studies with a new biosorbent ensis siliqua shell powder for removal two textile dyes from aqueous solution. JMES 8:1448–1459
Hu X, Fu X, Xu J, Wang C (2011) A novel layered organic polymer-inorganic hybrid zinc poly(styrene-phenylvinylphosphonate)-phosphate immobilized chiral salen Mn(III) catalyst large-scale asymmetric epoxidation of unfunctionalized olefins. J Org Chem 696:2797–2804
Hulvey Z, Falcao EHL, Eckert J, Cheetham AK (2009) Enhanced H2 adsorption enthalpy in the low-surface area, partially fluorinated coordination polymer Zn5(triazole)6 (tetrafluoroterephthalate)2 (H2O)2·4H2O. J Mater Chem 19:4307–4309
Jahagirdara AA, Zulfiqar Ahmed MN, Donappac N, Nagabhushanad H, Nagabhushanae BM (2014) Photocatalytic degradation of rhodamine B using nanocrystalline α-Fe2O3. J Mater Environ Sci 5:1426–1433
Jhang PC, Chuang NT, Wang SL (2010) Layered zinc phosphates with photoluminescence and photochromism: chemistry in deep eutectic solvents. Angew Chem Int Ed 49:4200–4204
Kaizra S, Louafi Y, Bellal B, Trari M, Rekhila G (2015) Electrochemical growth of tin(II) oxide films: application in photocatalytic degradation of methylene blue. Mater Sci Semicond Process 30:554–560
Lu YY, Zhang YY, Zhang J (2016) In situ loading of CuS nanoflowers on rutile TiO2 surface and their improved photocatalytic performance. Appl Surf Sci 370:312–319
Meziani D, Abdmeziem K, Bouacida S, Trari M, Merazig H (2016) Photo-electrochemical and physical characterizations of a new single crystal POM-based material Application to Rhodamine B photodegradation. Sol Energy Mater Sol Cells 147(2016):46–52
Ning ZL, Li WJ, Sun CY, Chen P, Chang ZD (2013) Synthesis and optical properties of zinc phosphate microspheres. Trans Nonferrous Met Soc China 23:718–724
Rajalakshmi S, Pitchaimuthu S, Kannan N, Velusamy P (2014) Enhanced catalytic activity of metal oxides/β-cyclodextrin nanocomposites for decoloration of rhodamine B dye under solar light irradiation. Appl Water Sci 1:115–127
Raliya R, Avery C, Chakrabarti S, Biswas P (2017) Photocatalytic degradation of methyl orange dye by pristine titanium, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl Nanosci 7:253–259
Roumila Y, Abdmeziem K, Rekhila G, Trari M (2016) Semiconducting properties of hydrothermally synthesized libethenite application to orange G photodegradation. Mater Sci Semicond Process 41:470–479
Ruzimuradov O, Sharipov K, Yarbekov A, Saidov K, Hojamberdiev M, Prasad RM, Cherkashinin G, Riedel R (2015) A facile preparation of dual-phase nitrogen-doped TiO2–SrTiO3 macroporous monolithic photocatalyst for organic dye photodegradation under visible light. J Eur Ceram Soc 35:1815–1821
Saranya M, Ramachandran R, Samuel EJJ, Jeongc S, Grace A (2015) Enhanced visible light photocatalytic reduction of organic pollutant and electrochemical properties of CuS catalyst. Powder Technol 279:209–220
Sikdar S, Pattanayek S, Ghorai TK (2017) Photocatalytic degradation of rhodamine B in water by visible light irradiated BMZ nanocomposite. Adv Mater Process 2:107–112
Sobahia TR, Amin MS, Mohameda RM (2017) Photocatalytic degradation of methylene blue dye by F-doped Co3O4 nanowires. Desalin Water Treat 74:346–353
Sun M, Li D, Chen Y, Chen W, Li W (2009) Synthesis and photocatalytic activity of calcium antimony oxide hydroxide for the degradation of dyes in water. J Phys Chem C 113:13825–13831
Tripathi B, Bhatt P, Chandra Kanth P, Yadav P, Desai B, Kumar Pandey M (2015) Temperature induced structural, electrical and optical changes in solution processed perovskite material: application in photovoltaics. Sol Energy Mater Sol Cells 132:615–622
Vijayakumar G, Tamilarasan R, Dharmendirakumar M (2012) Adsorption, kinetic, equilibrium and thermodynamic studies on the removal of basic dye rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J Mater Environ Sci 3:157–170
Wang M, Huang J, Tong Z, Li W, Chen J (2013) Reduced graphene oxide-cuprous oxide composite via facial deposition for photocatalytic dye-degradation. J Alloys Compd 568:26–35
Wang GM, Li JH, Zhang X, Jiao JQ, Bao ZZ, Zhao XM, Jiang WW, Wang YX, Lin JH (2014) Facile in situ syntheses of new templates and formations of three zinc phosphates. Inorg Chem Commun 46:295–300
Wang GM, Li JH, Gao CL, Zhang JC, Zhang X, Bao ZZ, Wang YX, Lin JH (2015) Synthesis and characterization of a novel inorganic-organic hybrid open-framework zinc phosphate with 16-ring channels. Solid State Sci 39:1–5
Weast RC (1978) Handbook of chemistry and physics, 58th edn. CRS Press Inc., New York
Xing Y, Li G, Meng H, Shi Z, Liu Y, Wei X, Yang Y, Pang W (2004) Synthesis and structure of a new layered zinc phosphate [C6N2H16]3.5[Zn14(PO4)7(HPO4)7] in the presence of trans-1,4-diaminocyclohexane, Inorg. Chem Commun 7:475–477
Yizhong C, Hongwei L, Li J, Guohe H, Li H (2016) Regional planning of new-energy systems within multi-period and multi-option contexts: a case study of Fengtai, Beijing, China. Renew Sustain Energy Rev 65:356–372
Zhang J, Yao ZG, Dai JC, Fu ZY (2013) A hybrid metal phosphate-phosphite materiel grafted with electron deficient organic components showing interesting fluorescent and photosensitive properties. J Mater Chem A 1:4945–4948
Acknowledgements
The authors thank Dr D. Meziani for collecting crystallography data and identification of as-synthesized compound and MHESR (Ministry of Higher Education and Scientific Research), Algeria, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Iskender Akkurt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bagtache, R., Abdmeziem, K., Dib, K. et al. Synthesis and photoelectrochemical characterization of KZn2(HPO4)PO4: application to rhodamine B photodegradation under solar light. Int. J. Environ. Sci. Technol. 16, 3819–3828 (2019). https://doi.org/10.1007/s13762-018-1883-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1883-8