Abstract
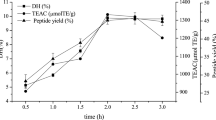
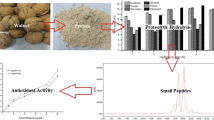
The aim of this study was to evaluate metal binding and antioxidant activities of hydrolyzed oat bran proteins followed by the determination of peptide sequences. Protamex oat bran protein hydrolysates (OBPH) were separated by reverse-phase HPLC into eight peptide fractions (F1–F8) and their abilities to either chelate metals (Fe2+, Ca2+) or prevent the oxidation of lipids were investigated. In the Fe2+ chelation assay, OBPH had significantly (p < 0.05) higher activity (39.7 %) than the best performed fraction F7 (22.8 %). The second most active was F5 with 12.1 % chelating activity and this was higher than the activity of the tripeptide glutathione (5.8 %) used as control. The two most Fe2+ chelating fractions (F5, F7) however had weak calcium binding (0.6–1.0 %) properties at peptide concentration ranging from 0.2 to 1.0 mg/mL. In the lipid peroxidation assay, OBPH and all HPLC fractions prevented the oxidation of linoleic acid. More than 60 peptides mainly derived from globulin and avenin proteins were identified using tandem mass spectrometry.





Similar content being viewed by others
References
Bao XL, Song M, Zhang J et al (2007) Calcium-binding ability of soy protein hydrolysates. Chin Chem Lett 18:1115–1118
Brieger K, Schiavone S, Miller FJ, Krause K-H (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659. doi:10.4414/smw.2012.13659
Cariolou MA, Morse DE (1988) Purification and characterization of calcium-binding conchiolin shell peptides from the mollusc, Haliotis rufescens, as a function of development. J Comp Physiol B 157:717–729. doi:10.1007/BF00691002
Chang Y-W, Alli I, Konishi Y, Ziomek E (2011) Characterization of protein fractions from chickpea (Cicer arietinum L.) and oat (Avena sativa L.) seeds using proteomic techniques. Food Res Int 44:3094–3104. doi:10.1016/j.foodres.2011.08.001
Chen HM, Muramoto K, Yamauchi F et al (1998) Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem 46:49–53. doi:10.1021/jf970649w
Chen N, Yang H, Sun Y et al (2012) Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 38:344–349
Chen D, Mu X, Huang H et al (2014) Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J Funct Foods 6:575–584
Gao Q, Smith JC, Tsopmo A (2014) Optimized Protamex digested oat bran proteins: antioxidant properties and identification of new peptides. Austin J Nutr Food Sci 2:1053
Girgih AT, Udenigwe CC, Hasan FM et al (2013) Antioxidant properties of Salmon (Salmo salar) protein hydrolysate and peptide fractions isolated by reverse-phase HPLC. Food Res Int 52:315–322
He X, Cao W, Zhao Z, Zhang C (2013) Analysis of protein composition and antioxidant activity of hydrolysates from Paphia undulate. J Food Nutr Res 1:30–36. doi:10.12691/jfnr-1-3-3
Hu FB, Willett WC (2002) Optimal diets for prevention of coronary heart disease. JAMA 288:2569–2578
Jiang B, Mine Y (2000) Preparation of novel functional oligophosphopeptides from hen egg yolk phosvitin. J Agric Food Chem 48:990–994
Jodayree S, Smith JC, Tsopmo A (2012) Use of carbohydrase to enhance protein extraction efficiency and antioxidative properties of oat bran protein hydrolysates. Food Res Int 46:69–75. doi:10.1016/j.foodres.2011.12.004
Jung WK, Kim S-K (2007) Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius belengerii) frame. Eur Food Res Technol 224:763–767. doi:10.1007/s00217-006-0371-4
Jung WK, Karawita R, Heo SJ et al (2006) Recovery of a novel Ca-binding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process Biochem 41:2097–2100
Kumar S (2011) Free radicals and antioxidants: human and food system. Adv Appl Sci Res 2:129–135
Lehtinen P, Kiiliainen K, Lehtomaki K, Laakso S (2003) Effect of heat treatment on lipid stability in processed oats. J Cereal Sci 37:215–221. doi:10.1006/jcrs.2002.0496
Liu F-R, Wang L, Wang R, Chen Z-X (2013) Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide-calcium complex. J Agric Food Chem 61:7537–7544. doi:10.1021/jf401868z
Markwell MAK, Haas SM, Bieber LL, Tolbert NE (1978) Modification of Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87:206–210. doi:10.1016/0003-2697(78)90586-9
McDermott A (2009) Bioactive peptides. Springer Science & Business Media, New York
Meisel H (2004) Multifunctional peptides encrypted in milk proteins. BioFactors 21:55–61
Pinchuk I, Shoval H, Dotan Y, Lichtenberg D (2012) Evaluation of antioxidants: scope, limitations and relevance of assays. Chem Phys Lipids 165:638–647. doi:10.1016/j.chemphyslip.2012.05.003
Prior R, Wu X, Kschaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4302
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
Rho SJ, Park S, Ahn C-W et al (2007) Dietetic and hypocholesterolaemic action of black soy peptide in dietary obese rats. J Sci Food Agric 87:908–913. doi:10.1002/jsfa.2808
Schneider CD, Reischak de Oliveira A (2009) Oxygen free radicals and exercise: mechanisms of synthesis and adaptation to the physical training. Rev Bras Med Esporte 10:314–318
Searle BC (2010) Scaffold: a bioinformatic tool for validating MS/MS-based proteomic studies. Proteomics 10:1265–1269. doi:10.1002/pmic.200900437
Shahidi F, Zhong Y (2010) Lipid oxidation and improving the oxidative stability. Chem Soc Rev 39:4067–4079
Vavrusova M, Skibsted LH (2013) Calcium binding to dipeptides of aspartate and glutamate in comparison with orthophosphoserine. J Agric Food Chem 61:5380–5384. doi:10.1021/jf400741e
Xie Z, Huang J, Xu X et al (2008) Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem 111:370–376
Zhu K, Zhou H, Qian H (2006) Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem 41:1296–1302. doi:10.1016/j.procbio.2005.12.029
Zhu L, Chen J, Tang X et al (2008) Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J Agric Food Chem 56:2714–2721. doi:10.1021/jf703697e
Acknowledgments
This work was supported by a Grant from National Science and Engineering Research Council of Canada (Grant No. 371908) and a Saudi Arabia government’s King Abdulah Foreign Scholarship to M. M. Baakdah. The authors thank Mr. Daniel Defoy from the Quebec Proteomics Platform available at the Quebec Genomics Center for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Baakdah, M.M., Tsopmo, A. Identification of peptides, metal binding and lipid peroxidation activities of HPLC fractions of hydrolyzed oat bran proteins. J Food Sci Technol 53, 3593–3601 (2016). https://doi.org/10.1007/s13197-016-2341-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2341-6