Abstract
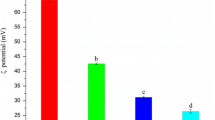
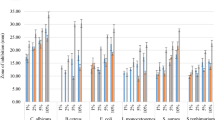
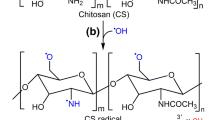
Chemically modified biofunctional chitosan derivatives may open up new applications in functional food and nutraceuticals development. Two new conjugates, namely vanillic acid and coumaric acid grafted chitosan derivatives were developed and comparative evaluation of their antioxidant and antimicrobial activities was carried out with ferulic acid and gallic acid grafted chitosan. The synthesized water soluble (1.0–4.5 mg/mL) chitosan-phenolic acid conjugates showed characteristic FTIR-spectroscopy band at around 1644 and 2933 cm−1. Free phenolic acid was not observed in TLC plate of the chitosan-phenolic acid conjugates and UV–vis spectra showed primary absorption peak of the corresponding phenolic acids confirming the grafting reaction. Total antioxidant activity of the chitosan-phenolic acid derivatives ranged from ~12 to 29 g ascorbic acid equivalent/100 g of the conjugate. Minute quantity of the derivatives (~57–162 μg and 82–109 μg respectively) could give 50 % inhibition of 2, 2′-diphenyl-1-picrylhydrazyl radical and hydroxyl free radical. Broad spectrum antibacterial activity was observed for all four derivatives against an array of foodborne pathogens and spoilage bacteria. Coumaric acid grafted chitosan showed promise as a food preservative as it inhibited the growth of several foodborne pathogens and spoilage bacteria, including Staphylococcus aureus. Among all the four derivatives, ferulic acid and gallic acid grafted chitosan had lowest minimum inhibitory concentration against Staphylococcus aureus and Pseudomonas aeruginosa respectively.




Similar content being viewed by others
References
Aytekin AO, Morimura S, Kida K (2011) Synthesis of chitosan–caffeic acid derivatives and evaluation of their antioxidant activities. J Biosci Bioeng 111:212–216
Bobu E, Nicu R, Lupei M, Ciolacu FL, Desbrières J (2011) Synthesis and characterization of n-alkyl chitosan for papermaking applications. Cellul Chem Technol 45:619–625
Božič M, Gorgieva S, Kokol V (2012) Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr Polym 87:2388–2398
Casettari L, Gennari L, Angelino D, Ninfali P, Castagnino E (2012) ORAC of chitosan and its derivatives. Food Hydrocoll 28:243–247
Cho Y, Kim S, Ahn C, Je J (2011) Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr Polym 83:1617–1622
CLSI (2012) Performance standards for antimicrobial disk susceptibility tests; approved standard—eleventh edition, Vol 32 (1). Clinical and Laboratory Standards Institute, Wayne, 58p
Curcio M, Puoci F, Iemma F, Parisi OI, Cirillo G, Spizzirri UG, Picci N (2009) Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J Agric Food Chem 57:5933–5938
Ferguson LR, Zhu S, Harris PJ (2005) Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol Nutr Food Res 49:585–593
Gitzinger M, Kemmer C, Fluri DA, ElBaba MD, Weber W, Fussenegger M (2012) The food additive vanillic acid controls transgene expression in mammalian cells and mice. Nucleic Acids Res 40:37–50
Gombas DE, ChenY CRS, Scott VN (2003) Survey of Listeria monocytogenes in ready-to-eat foods. J Food Prot 66:559–569
Halliwell B, Gutteridge JMC, Aruoma OI (1987) The deoxyribose method: a simple “test tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219
Kamil J, Jeon Y, Shahidi F (2002) Antioxidative activity of chitosans of different viscosity in cooked comminuted flesh of herring (clupea harengus). Food Chem 79:69–77
Karunasagar I, Karunasagar I (2000) Listeria in tropical fish and fishery products. Int J Food Microbiol 62:177–181
Lee DS, Woo JY, Ahn CB, Je JY (2014) Chitosan–hydroxycinnamic acid conjugates: preparation, antioxidant and antimicrobial activity. Food Chem 148:97–104
Liu J, Lu J, Kan J, Jin C (2013) Synthesis of chitosan-gallic acid conjugate: structure characterization and in vitro anti-diabetic potential. Int J Biol Macromol 62:321–329
Liu J, Wen X, Lu J, Kan J, Jin C (2014) Free radical mediated grafting of chitosan with caffeic and ferulic acids: structures and antioxidant activity. Int J Biol Macromol 65:97–106
Mancuso C, Santangelo R (2014) Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol 65:185–195
Pasanphan W, Buettner GR, Chirachanchai S (2010) Chitosan gallate as a novel potential polysaccharide antioxidant: an EPR study. Carbohydr Res 345:132–140
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Prince PSM, Roy AJ (2013) p-Coumaric acid attenuates apoptosis in isoproterenol-induced myocardial infarcted rats by inhibiting oxidative stress. Int J Cardiol 168:3259–3266
Prince PSM, Rajakumar S, Dhanasekar K (2011) Protective effects of vanillic acid on electrocardiogram, lipidperoxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol 668:233–240
Sabnis S, Block LH (2000) Chitosan as an enabling excipient for drug delivery systems. I. Molecular modifications. Int J Biol Macromol 27:181–186
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324
Schreiber SB, Bozell JJ, Hayes DG, Zivanovic S (2013) Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll 33:207–214
Shahidi F, Kamil J, Jeon Y, Kim S (2002) Antioxidant role of chitosan in a cooked cod (gadus mornua) model system. J Food Lipids 9:57–64
Stojković D, Petrović J, Soković M, Glamočlija J, Kukić-Marković J, Petrović S (2013) In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J Sci Food Agric 93:3205–3208
Sun X, Wang Z, Kadouh H, Zhou K (2014) The antimicrobial, mechanical, physical and structural properties of chitosan-gallic acid films. LWT Food Sci Technol 57:83–89
Takahasi H, Kashimura M, Koiso H, Kuda T, Kimura B (2013) Use of ferulic acid as a novel candidate of growth inhibiting agent against Listeria monocytogenes in ready-to-eat food. Food Control 33:244–248
Woranuch S, Yoksan R (2013) Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr Polym 96:495–502
Xie W, Xu P, Liu Q (2001) Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett 11:1699–1701
Yu SH, Mi FL, Pang JC, Jiang SC, Kuo TH, Wu SJ, et al (2011) Preparation and characterization of radical and pH-responsive chitosan-gallic acid conjugate drug carriers. Carbohydr Polym 84:794–802
Acknowledgments
The authors sincerely acknowledge analytical services provided by “Sophisticated test and instrumentation centre” of Cochin University of Science and Technology, Cochin, Kerala, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Niladri Sekhar Chatterjee, Satyen Kumar Panda and Mary Navitha contributed equally.
Highlights
First report of vanillic acid and coumaric acid functionalization of chitosan
Higher grafting ratio of phenolic acids on chitosan
Better antioxidant activity than earlier reports
Broad spectrum antibacterial activity against foodborne pathogens Potential application in functional food
Rights and permissions
About this article
Cite this article
Chatterjee, N.S., Panda, S.K., Navitha, M. et al. Vanillic acid and coumaric acid grafted chitosan derivatives: improved grafting ratio and potential application in functional food. J Food Sci Technol 52, 7153–7162 (2015). https://doi.org/10.1007/s13197-015-1874-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1874-4