Abstract
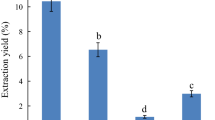
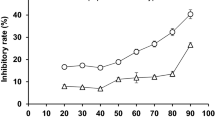
The oil in mackerel muscle was extracted using an environmental friendly solvent, supercritical carbon dioxide (SC-CO2) at a semi-batch flow extraction process and an n-hexane. The SC-CO2 was carried out at temperature 45 °C and pressures ranging from 15 to 25 MPa. The flow rate of CO2 (27 g/min) was constant at the entire extraction period of 2 h. The highest oil extracted residues after SC-CO2 extraction was used for activity measurement of digestive enzymes. Four digestive enzymes were found in water soluble extracts after n-hexane and SC-CO2 treated samples. Amylase, lipase and trypsin activities were higher in water soluble extracts after SC-CO2 treated samples except protease. Among the four digestive enzymes, the activity of amylase was highest and the value was 44.57 uM/min/mg of protein. The water soluble extracts of SC-CO2 and n-hexane treated mackerel samples showed same alkaline optimum pH and pH stability for each of the digestive enzymes. Optimum temperature of amylase, lipase, protease and trypsin was 40, 50, 60 and 30 °C, respectively of both extracts. More than 80 % temperature stability of amylase, lipase, protease and trypsin were retained at mentioned optimum temperature in water soluble extracts of both treated samples. Based on protein patterns, prominent protein band showed in water soluble extracts after SC-CO2 treated samples indicates no denaturation of protein than untreated and n-hexane.








Similar content being viewed by others
References
Abdelkarder AN, Kim SB, Lee YB, Chun BS (2012) Digestive enzymes characterization of krill (Euphausia superba) residues deoiled by supercritical carbon dioxide and organic solvents. J Ind Eng Chem 18:1314–1319
Aryee ANA, Simpson BK, Villalonga R (2007) Lipase fraction from the viscera of grey mullet (Mugil cephalus): isolation, partial purification, and some biochemical characteristics. J Enzym Microb Technol 40:394–402
Bergmeyer HU, Gawehn K, Grassi M (1974) In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, Inc., New York, pp 515–516
Bezerra RS, Lins EJF, Alencar RB, Paiva PMG, Chaves MEC, Coelho LCBB, Carvalho LB (2005) Alkaline proteinase from intestine of nile tilapia (Oreochromis niloticus). J Process Biochem 40:1829–1834
Dendinger JE, O’connor KL (1990) Purification and characterization of a trypsin-like enzyme from the midgut gland of the Atlantic blue crab, Callinectes sapidus. J Comp Biochem Physiol B 95:525–530
Djamel C, Ali T, Nelly C (2009) Acid protease production by isolated species of penicillium. Eur J Sci Res 25(3):469–477
Eshel A, Lindner P, Smirnoff P, Newton S, Harpaz S (1993) Comparative study of proteolytic enzymes in the digestive tracts of the European sea bass and hybrid striped bass reared in freshwater. J Comp Biochem Physiol A 106:627–634
Espósito TS, Amaral IPG, Buarque DS, Oliveira GB, Carvalho LB Jr, Bezerra RS (2009) Fish processing waste as a source of alkaline proteases for laundry detergent. J Food Chem 112:125–130
Ge Y, Ni Y, Chen Y, Cai T (2002) Optimization of the supercritical fluid extraction of natural vitamin E from wheat germ using response surface methodology. J Food Sci 67:239–243
Hidalgo MC, Urea E, Sanz A (1999) Comparative study of digestive enzymes in fish with different nutritional habits: proteolytic and amylase activities. J Aquac 170:267–283
Karun T, Uthaiwan K, Arunee E, Krisna RT (2010) Temperature and pH characteristics of amylase and lipase at different developmental stages of Siamese fighting fish (Betta splendens regan, 1910). Kasetsart J (Nat Sci) 44:210–219
Klomklao S, Benjakul S, Visessnguan W, Kishimura H, Simpson BK (2006) Purification and characterization of trypsin from the spleen of tongol tuna (Thunnus tonggol). J Agric Food Chem 54:5617–5622
Kubrak OI (2007) Microbe amylases: characteristic, properties and practical use. J Microb Z 69(6):56–76
Kumar S, Kikon K, Upadhyay A, Kanwar SS, Gupta R (2005) Production, purification, and characterization of lipase from thermophilic and alkaliphilic Bacillus coagulans BTS-3. J Protein Expr Purif 41:38–44
Laemmli UK (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Roserbrough NJ, Farr AW, Randall RJ (1951) Protein measurements with pholin phenol reagent. J Biol Chem 193:265–275
Martinez A, Olsen RL, Serra JL (1988) Purification and characterization of two trypsin-like enzymes from the digestive tract of anchovy Engraulis encrasicholus. J Comp Biochem Physiol B 91:677–684
Mendes RL, Nobre BP, Cardoso MT, Pereire AP, Palavre AF (2003) Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. J Inorg Chim Acta 356:328–334
Miller GL (1959) Use of dinitrosalicyclic acid reagent for the determination of reducing sugar. J Anal Chem 31:426–429
Morita A, Kajimoto O (1990) Solute solvent interaction in nonpolar supercritical fluid: a clustering model and size distribution. J Phys Chem 94:6420–6425
Murakami K, Noda M (1981) Studies on proteinases from the digestive organs of sardine-purification and characterization of three alkaline proteinases from the pyloric ceca. J Biochim Biophys Acta B 65:17–26
Natalia Y, Hashim R, Ali A, Chong A (2004) Characterization of digestive enzymes in a carnivorous ornamental fish, the Asian bony tongue Scleropages for mosus (Osteoglossidae). J Aquacultur 233:305–320
Noman ASM, Hoque MA, Sen PK, Karim MR (2006) Purification and some properties of amylase from post-harvest Pachyrhizus erosus L. tuber. J Food Chem 99:444–449
Oda K, Murao S (1974) Purification and properties of carboxyl proteinase in basidiomycetes. J Agric Biol Chem 38:2435–2437
Ogino H, Miyamoto K, Ishikawa H (1994) Organic solvent tolarent bacteria which secretes organic solvent stable lipolytic enzyme. J Appl Env Microb 60(10):3884–3886
Pariser ER, Wallerstei, MB, Corkery CJ, Brown NL (1978) Fish protein concentrate: Panacea for world Malnutrition. MIT Press, Boston, ISBN: 10: 0262160692, pp 256
Park JY, Lee MK, Uddin MS, Chun BS (2008) Removal of off flavors and isolation of fatty acids from boiled anchovies using supercritical carbon dioxide. J Biotechnol Bioproc Eng 13:298–303
Parsertsan P, Jitbunjerdkul S, Trairatananukoon Prachumratana T (2001) Production of enzyme and protein hydrolyzate from fish processing waste. In: Roussos S, Soccol CR, Pandey A, Augur C (eds) New horizons in biotechnology. Kluwer Academic Publisher, India, pp 63–72
Perona JJ, Craick SC (1995) Structural basis of substrate specificity in the serine proteases. Protein Sci 4(3):337–360
Pham V, Soottawat B (2006) Purification and characterization of trypsin from pyloric caeca of bigeye snapper (pricanthus macracanthus). J Food Biochem 30:478–495
Poonsuk P, Thiraratana P (2008) Properties of protease and lipase from whole and individual organ of viscera from three tuna species. Songklanakarin J Sci Technol 30:77–86
Shahidi F, Janak-Kamil YVA (2001) Enzymes from fish and aquatic invertebrates and their application in the food industry. J Trends Food Sci Technol 12:435–464
Simpson BK, Haard NF (1984) Trypsin from Greenland cod, Gadus ogac. Isolation and comparative properties. J Comp Biochem Physiol B 79:613–622
Simpson BK, Smith JP, Haard NF (1991) Marine enzymes. In: Hui YH (ed) Encyclopedia of food science and technology. Wiley, New York, pp 1645–1653
Sun M, Temelli F (2006) Supercritical carbon dioxide extractions of carotenoids from carrot using canola oil as a continuous co-solvent. J Supercrit Fluids 37:397–408
Temelli F, Leblanc E, Fu L (1995) Supercritical carbon dioxide extraction of oil from Atlantic Mackerel (Scomber scombrus) and protein functionality. J Food Sci 60:703–706
Uddin MS, Ahn HM, Kishimura H, Chun BS (2009) Comparative study of digestive enzymes of squid (Todarodes pacificus) Viscera after supercritical carbon dioxide and organic solvent extraction. J Biotechnol Bioproc Eng 14:338–344
Vilhelmsson O (1997) The state of enzyme biotechnology in the fish processing industry. J Trends Food Sci Technol 8:266–270
Vulfson EN (1994) Industrial applications of lipases. In: Wooley P, Petersen SB (eds) Lipases. Cambridge University Press, Cambridge, pp 271–288
Yamaguchi K, Murakami M, Nakano H, Konosu S, Kokura T, Yamamoto H, Kosaka M, Hata K (1986) Supercritical carbon dioxide extraction of oils from Antarctic krill. J Agric Food Chem 34:904–907
Acknowledgments
This work (Grants No. C0019011) was supported by Business for Academic-industrial Cooperative establishments funded Korea Small and Medium Business Administration in 2012
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asaduzzaman, A.K.M., Chun, BS. Characterization of digestive enzymes from de-oiled mackerel (Scomber japonicus) muscle obtained by supercritical carbon dioxide and n-hexane extraction as a comparative study. J Food Sci Technol 52, 3494–3503 (2015). https://doi.org/10.1007/s13197-014-1408-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1408-5