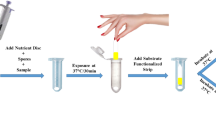
Abstract
The spore forming Bacillus cereus (66) was screened for the induction of β-lactamase in presence of an inducer using iodometric assay. A significant induction in marker enzyme was observed in B. cereus 66 at maximum residual limit (MRL) of penicillin, ampicillin, cloxacillin, amoxicillin, cefalexin, and cephazolin belonging to β-lactam group of antibiotics. A microbial based assay, where enzyme induction was optimized at pH 7.0, temperature 30°C, and whey powder (0.25%) after 4 h of incubation. The spore based assay was tested with milk samples spiked with 6 different β-lactam antibiotics. The results were 100 and 83.33% in correlation with microbial receptor and inhibition based assay, respectively. Overall, spore based assay can be a useful and cost effective tool for the specific detection of β-lactam group of antibiotics in milk.

Similar content being viewed by others
References
Ahmad S, Yadava JNS (1979) Rapid detection of β-lactam antibiotic resistance among clinical isolates of Escherichia.coli. Indian Vet Med J 32:256–259
Aureli P, Ferrini AM, Mannoni V (1996) Presumptive identification of sulphonamide and antibiotic residues in milk by microbial inhibitor tests. Food Control 7(3):165–168
Catlin BW (1975) Iodometric detection of Haemophiluc influenzae β–lactamase: rapid presumptive test for Ampicillin resistance. Antimicrob Agents Chemother 7:265–270
Chen Y, Succi J, Tenover FC, Koehler TM (2003) β-lactamase genes of the penicillin-susceptible bacillus anthracis sterne strain. J Bacteriol 185(3):823–830
Davis BR, Abraham EP, Melling J (1974) Seperation, purification and properties of β-Lactamases-I and β-Lactamases-II from Bacillus cereus 569/H/9. Biochem J 143:115–127
Downes FP, Ito K (2001) Compendium of methods for the microbiological examination of foods. 4th Edition. In: Downes FP, Ito K (eds) American Public Health Association, Washington DC
Drobniewski FA (1993) Bacillus cereus and related species. Clin Microbiol Rev 6(4):324–338
EC (1990) Council regulation 2377/90/EU of 26 June 1990 laying down a community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Offic J Euro Union L224:1–8
EC (2010) Council regulation 37/2010/EU of 22nd December 2009 on pharmacologically active substances and their classification regarding maximum residues limits in foodstuffs of animal origin. Offic J Euro Union L15:1–72
Fenselau C, Crystal H, Nuttinee T, Steve S, Olli L, Nathan E (2007) Identification of β-Lactamase in Antibiotic-resistant Bacillus cereus spores. Appl Environ Microbiol 74(3):904–906
Fonze E, Vanhove M, Dive G, Sauvage E, Frere JM, Charlier P (2002) Crystal structures of the Bacillus licheniformis BS3 class A β-lactamase and of the acyl-enzyme adduct formed with cefoxitin. Biochem 41:1877–1885
IDF (1991) Detection and confirmation of inhibitors in milk and milk products. International Dairy Federation Bulletin; 258. International Dairy Federation, Brussels
Imsande J (1970) Regulation of penicillinase synthesis: evidence for a unified model. J Bacteriol 101:173–180
Imsande J, Zyskind JW, Mile L (1972) Regulation of staphylococcal penicillinase synthesis. J Bacteriol 109:122–133
Kabir J, Umohb JC, Audu-okoha E, Umoha JU, Kwaga JKP (2004) Veterinary drug use in poultry farms and determination of antimicrobial drug residues in commercial eggs and slaughtered chiken in Kaduna state, Nigeria. Food Control 15:99–105
Katz SE, Brady MS (2000) Antibiotic residues in food and their significance. Food Biotechnol 14(3):147–171
Kirbiš A (2007) Microbiological screening method for detection of aminoglycosides, β-lactames, macrolides, Tetracyclines and quinolones in meat samples. Slov Vet Res 44(1/2):11–18
Kogut M, Pollock MR, Tridgell EJ (1956) Purification of penicillin-induced penicillinase of B.cereus NRRL 569: a comparison of its properties with those of a similarly purified penicillinase produced spontaneously by a constitutive mutant strain. Biochem J 62:391–401
Kotiranta A, Lounatmaa K, Haapasalo M (2000) Epidemiology and pathogenesis of Bacillus cereus infections. Microb Infect 2:189–198
Livermore DM, Brown DFJ (2001) Detection of β-lactamase-mediated resistance. J Antimicrob Chemother 48:59–64
Mayra-Makinen A (1995) Technological significance of residues for the dairy industry. Bull Intern Dairy Fed 9505:136–143
Navrátilová P (2008) Screening methods used for the detection of veterinary drug residues in raw cow milk – a review. Czech J Food Sci 26:393–401
Pikkemaat MG (2009) Microbial screening methods for detection of antibiotic residues in slaughter animals. Anal Bioanal Chem 395:893–905
Pikkemaat MG, Sabrina OD, Jan S, Michel R, van Egmond HJ (2008) A new microbial screening method for the detection of antimicrobial residues in slaughter animals: the Nouws antibiotic test (NAT-screening). Food Control 19:781–789
Pollock MR (1950) Penicillinase adaptation in B. cereus: adaptive enzyme formation in the absence of free substrate. Br J Exp Pathol 31:739–753
Pollock MR (1956) The cell-bound penicillinase of Bacillus cereus. J Gen Microbiol 15:154–169
Pollock MR (1961) The measurement of the liberation of penicillinase from Bacillus subtilis (i.e. B. licheniformis 6346). J Gen Microbiol 26:239–253
Pollock MR (1965) Purification and properties of penicillinase from two strains of Bacillus licheniformis: a chemical, physiochemical and physiological comparison. Biochem J 94:666–675
Shah R, Day RA (1963) Comparative studies of penicillinase induction in microorganisms by natural and semi-synthetic penicillins. Ohio J Sci 63:217–224
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. The Iowa State University Press, Iowa
Wong W, Au H, Yap H, Leung Y, Wong K, Zhao Y (2011) Structural studies of the mechanisms for biosensing antibiotics in a fluorescein labeled β-lactamase. BMC Struct Biol 11:15. doi:10.1186/1472-6807-11-15
Yildiz Ö, Unluturk S (2009) Differential scanning calorimetry as a tool to detect antibiotic residues in ultra high temperature whole milk. Int J Food Sci Technol 44(12):2577–2582
Zvirdauskiene R, Salomskiene J (2007) An evaluation of different microbial and rapid tests for determining inhibitors in milk. Food Control 18:541–547
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, S., Kumar, N., Vishweswaraiah, R.H. et al. Microbial based assay for specific detection of β-lactam group of antibiotics in milk. J Food Sci Technol 51, 1161–1166 (2014). https://doi.org/10.1007/s13197-011-0609-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0609-4