Abstract
Apoptosis plays an important role in both carcinogenesis and cancer treatment. Understanding the mechanisms through which resistance to apoptosis occurs in cancer cells has huge implications for cancer treatment. Although pieces of evidence have shown that elevated levels of global O-GlcNAcylation play an anti-apoptotic role in myriad cancers, the underlying mechanism is still ambiguous. In this study, we demonstrated that FOXA2, an essential transcription factor for liver homeostasis and hepatocellular carcinoma (HCC) development, inhibits doxorubicin (DOX)-induced apoptosis through elevating cellular O-GlcNAcylation in HCC cells. In response to DOX treatment, elevated FOXA2 and global O-GlcNAcylation level was observed in HCC cells, and higher FOXA2 levels indicated lower levels of DOX-induced apoptosis. Subsequently, we demonstrated that FOXA2 is a direct transcriptional activator of the hexosamine biosynthetic pathway (HBP) rate-limiting enzyme GFPT1. The upregulation of FOXA2 expression induced the synthesis of intracellular UDP-GlcNAc, which is the sugar substrate of O-GlcNAcylation produced by the HBP. The flux through the HBP elevated the global O-GlcNAcylation level and led to the activation of survival signaling pathways in HCC cells. Furthermore, GFPT1 was proved to be an important downstream regulator of FOXA2-mediated apoptotic suppression. These results provide insights into the molecular mechanism by which FOXA2 inhibits DOX-induced HCC cell apoptosis and suggest that targeting FOXA2 might offer a new strategy for HCC treatment.






Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH (2008) Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med 14(8):828–836. https://doi.org/10.1038/nm.1853
Burt D, Brodbeck K, Haring HU, Schleicher ED, Weigert C (2005) Partial characterisation of the human GFAT promoter: effect of single nucleotide polymorphisms on promoter function. Biochim Biophys Acta 1740(1):85–90. https://doi.org/10.1016/j.bbadis.2005.01.007
Butkinaree C, Park K, Hart GW (2010) O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 1800(2):96–106. https://doi.org/10.1016/j.bbagen.2009.07.018
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS et al (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16(25):3267–3285. https://doi.org/10.2174/092986709788803312
Fathi N, Rashidi G, Khodadadi A, Shahi S, Sharifi S (2018) STAT3 and apoptosis challenges in cancer. Int J Biol Macromol 117:993–1001. https://doi.org/10.1016/j.ijbiomac.2018.05.121
Hanover JA, Chen W, Bond MR (2018) O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle. J Bioenerg Biomembr 50(3):155–173. https://doi.org/10.1007/s10863-018-9751-2
Jindal A, Thadi A, Shailubhai K (2019) Hepatocellular carcinoma: etiology and current and future drugs. J Clin Exp Hepatol 9(2):221–232. https://doi.org/10.1016/j.jceh.2019.01.004
Lee SJ, Kwon OS (2020) O-GlcNAc Transferase inhibitor synergistically enhances doxorubicin-induced apoptosis in HepG2 cells. Cancers (Basel) 12(11):3154. https://doi.org/10.3390/cancers12113154
Lee CS, Friedman JR, Fulmer JT, Kaestner KH (2005) The initiation of liver development is dependent on Foxa transcription factors. Nature 435(7044):944–947. https://doi.org/10.1038/nature03649
Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K et al (2012) Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol 35(5):980–985. https://doi.org/10.1007/s00270-011-0287-7
Lin L, Liang H, Wang Y, Yin X, Hu Y, Huang J et al (2014) microRNA-141 inhibits cell proliferation and invasion and promotes apoptosis by targeting hepatocyte nuclear factor-3β in hepatocellular carcinoma cells. BMC Cancer 14:879. https://doi.org/10.1186/1471-2407-14-879
Lin J, Zhang D, Fan Y, Chao Y, Chang J, Li N et al (2018) Regulation of cancer stem cell self-renewal by HOXB9 antagonizes endoplasmic reticulum stress-induced melanoma Cell apoptosis via the miR-765-FOXA2 Axis. J Invest Dermatol 138(7):1609–1619. https://doi.org/10.1016/j.jid.2018.01.023
Liu Y, Qin Z, Cai L, Zou L, Zhao J, Zhong F (2017) Selection of internal references for qRT-PCR assays of human hepatocellular carcinoma cell lines. Biosci Rep 37(6):BSR20171281. https://doi.org/10.1042/BSR20171281
Liu TW, Zandberg WF, Gloster TM, Deng L, Murray KD, Shan X et al (2018) Metabolic inhibitors of O-GlcNAc transferase that act in vivo implicate decreased O-GlcNAc levels in leptin-mediated nutrient sensing. Angew Chem Int Ed Engl 57(26):7644–7648. https://doi.org/10.1002/anie.201803254
Liu Y, Cao Y, Pan X, Shi M, Wu Q, Huang T et al (2018) O-GlcNAc elevation through activation of the hexosamine biosynthetic pathway enhances cancer cell chemoresistance. Cell Death Dis 9(5):485. https://doi.org/10.1038/s41419-018-0522-0
Ma Z, Vocadlo DJ, Vosseller K (2013) Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem 288(21):15121–15130. https://doi.org/10.1074/jbc.M113.470047
Mas VR, Maluf DG, Archer KJ, Yanek K, Kong X, Kulik L, Freise CE, Olthoff KM, Ghobrial RM, Mclver P, Fisher R (2009) Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med 15(3-4):85–94. https://doi.org/10.2119/molmed.2008.00110
Peternelj TT, Marsh SA, Strobel NA, Matsumoto A, Briskey D, Dalbo VJ et al (2015) Glutathione depletion and acute exercise increase O-GlcNAc protein modification in rat skeletal muscle. Mol Cell Biochem 400(1–2):265–275. https://doi.org/10.1007/s11010-014-2283-0
Schug J (2008) Using TESS to predict transcription factor binding sites in DNA sequence. Curr Protoc Bioinformatics. https://doi.org/10.1002/0471250953.bi0206s21
Taylor RP, Geisler TS, Chambers JH, McClain DA (2009) Up-regulation of O-GlcNAc transferase with glucose deprivation in HepG2 cells is mediated by decreased hexosamine pathway flux. J Biol Chem 284(6):3425–3432. https://doi.org/10.1074/jbc.M803198200
Villanueva A (2019) Hepatocellular Carcinoma. N Engl J Med 380(15):1450–1462. https://doi.org/10.1056/NEJMra1713263
von Meyenn F, Porstmann T, Gasser E, Selevsek N, Schmidt A, Aebersold R et al (2013) Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab 17(3):436–447. https://doi.org/10.1016/j.cmet.2013.01.014
Walter LA, Lin YH, Halbrook CJ, Chuh KN, He L, Pedowitz NJ et al (2020) Inhibiting the hexosamine biosynthetic pathway lowers O-GlcNAcylation levels and sensitizes cancer to environmental stress. Biochemistry 59(34):3169–3179. https://doi.org/10.1021/acs.biochem.9b00560
Wang K (2015) Molecular mechanisms of hepatic apoptosis regulated by nuclear factors. Cell Signal 27(4):729–738. https://doi.org/10.1016/j.cellsig.2014.11.038
Wang K, Brems JJ, Gamelli RL, Holterman AX (2013) Foxa2 may modulate hepatic apoptosis through the cIAP1 pathway. Cell Signal 25(4):867–874. https://doi.org/10.1016/j.cellsig.2012.12.012
Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR et al (2014) Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 156(6):1179–1192. https://doi.org/10.1016/j.cell.2014.01.014
Wang B, Liu G, Ding L, Zhao J, Lu Y (2018) FOXA2 promotes the proliferation, migration and invasion, and epithelial mesenchymal transition in colon cancer. Exp Ther Med 16(1):133–140. https://doi.org/10.3892/etm.2018.6157
Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V et al (2000) TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res 28(1):316–319. https://doi.org/10.1093/nar/28.1.316
Wolfrum C, Besser D, Luca E, Stoffel M (2003) Insulin regulates the activity of forkhead transcription factor Hnf-3beta/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc Natl Acad Sci U S A 100(20):11624–11629. https://doi.org/10.1073/pnas.1931483100
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. https://doi.org/10.1186/1756-9966-30-87
Xu C, Liu GD, Feng L, Zhang CH, Wang F (2018) Identification of O-GlcNAcylation modification in diabetic retinopathy and crosstalk with phosphorylation of STAT3 in retina vascular endothelium cells. Cell Physiol Biochem 49(4):1389–1402. https://doi.org/10.1159/000493444
Zachara NE, Hart GW (2004) O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta 1673(1–2):13–28. https://doi.org/10.1016/j.bbagen.2004.03.016
Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW (2004) Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279(29):30133–30142. https://doi.org/10.1074/jbc.M403773200
Zachara NE, Molina H, Wong KY, Pandey A, Hart GW (2011) The dynamic stress-induced “O-GlcNAc-ome” highlights functions for O-GlcNAc in regulating DNA damage/repair and other cellular pathways. Amino Acids 40(3):793–808. https://doi.org/10.1007/s00726-010-0695-z
Zhang J, Zhang Z, Sun J, Ma Q, Zhao W, Chen X et al (2019) MiR-942 regulates the function of breast cancer cell by targeting FOXA2. Biosci Rep 39(11):BSR20192298. https://doi.org/10.1042/BSR20192298
Zhao Y, Butler EB, Tan M (2013) Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 4:e532. https://doi.org/10.1038/cddis.2013.60
Funding
This work was supported by grants from the Natural Science Foundation of China (31870793, 31971214), Natural Science Foundation of Liaoning Province (2019-MS-042), and the Fundamental Research Funds for the Central Universities (DUT20YG130, DUT20YG116).
Author information
Authors and Affiliations
Contributions
Huang H conceived and designed the study. Huang H, Wang Y, Huang T, Wang L, Liu Yangzhi, Wu Q, Yu A, Shi M, and Wang X performed the experiments. Huang H and Liu Yubo wrote the paper. Li W and Zhang J reviewed and edited the manuscript. All authors read and approved the manuscript. The authors declared that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
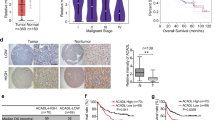
Key Points FOXA2 is a direct transcriptional activator of the HBP rate-limiting enzyme GFPT1. FOXA2 promotes the synthesis of UDP-GlcNAc and thus elevates cellular O-GlcNAcylation. FOXA2 inhibits DOX-induced apoptosis by elevating O-GlcNAcylation in HCC cells.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, H., Wang, Y., Huang, T. et al. FOXA2 inhibits doxorubicin-induced apoptosis via transcriptionally activating HBP rate-limiting enzyme GFPT1 in HCC cells. J Physiol Biochem 77, 625–638 (2021). https://doi.org/10.1007/s13105-021-00829-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00829-6