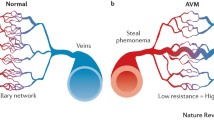
Abstract
Patients harboring brain arteriovenous malformation (bAVM) are at life-threatening risk of rupture and intracranial hemorrhage (ICH). The pathogenesis of bAVM has not been completely understood. Current treatment options are invasive, and ≈ 20 % of patients are not offered interventional therapy because of excessive treatment risk. There are no specific medical therapies to treat bAVMs. The lack of validated animal models has been an obstacle for testing hypotheses of bAVM pathogenesis and testing new therapies. In this review, we summarize bAVM model development and bAVM pathogenesis and potential therapeutic targets that have been identified during model development.






Similar content being viewed by others
Abbreviations
- bAVM:
-
Brain arteriovenous malformation
- ICH:
-
Intracranial hemorrhage
- HHT:
-
Hereditary hemorrhagic telangiectasia
- ENG:
-
Endoglin
- ALK1:
-
Activin-like kinase 1
- VEGF:
-
Vascular endothelial growth factor
- PDGFB:
-
Platelet-derived growth factor-B
- AV:
-
shunt Arteriovenous shunt
- f:
-
Allele with two loxP sites flanking the target sequence
References
Young WL. Intracranial arteriovenous malformations: pathophysiology and hemodynamics (Chapter 6). In: Jafar JJ, Awad IA, Rosenwasser RH, editors. Vascular Malformations of the Central Nervous System. New York: Lippincott Williams & Wilkins; 1999. p. 95–126.
Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359(9309):863–73.
Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340(23):1812–8.
Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98(1):3–7.
Bambakidis NC, Cockroft K, Connolly ES, Amin-Hanjani S, Morcos J, Meyers PM, et al. Preliminary results of the ARUBA sudy. Neurosurgery. 2013;73(2):E379–81.
Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614–21.
Rubin D, Santillan A, Greenfield JP, Souweidane M, Riina HA. Surgical management of pediatric cerebral arteriovenous malformations. Childs Nerv Syst. 2010;26(10):1337–44.
Potter CA, Armstrong-Wells J, Fullerton HJ, Young WL, Higashida RT, Dowd CF, et al. Neonatal giant pial arteriovenous malformation: genesis or rapid enlargement in the third trimester. J Neurointerv Surg. 2009;1(2):151–3.
Du R, Hashimoto T, Tihan T, Young WL, Perry VH, Lawton MT. Growth and regression of an arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia: case report. J Neurosurg. 2007;106(3):470–7.
Hino A, Fujimoto M, Iwamoto Y, Takahashi Y, Katsumori T. An adult case of recurrent arteriovenous malformation after "complete" surgical excision: a case report. Surg Neurol. 1999;52(2):156–8.
Kader A, Goodrich JT, Sonstein WJ, Stein BM, Carmel PW, Michelsen WJ. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg. 1996;85(1):14–8.
Klimo Jr P, Rao G, Brockmeyer D. Pediatric arteriovenous malformations: a 15-year experience with an emphasis on residual and recurrent lesions. Childs Nerv Syst. 2007;23(1):31–7.
Lindqvist M, Karlsson B, Guo WY, Kihlstrom L, Lippitz B, Yamamoto M. Angiographic long-term follow-up data for arteriovenous malformations previously proven to be obliterated after gamma knife radiosurgery. Neurosurgery. 2000;46(4):803–8.
Inoue S, Liu W, Inoue K, Mineharu Y, Takenaka K, Yamakawa H, et al. Combination of linkage and association studies for brain arteriovenous malformation. Stroke. 2007;38(4):1368–70.
Bharatha A, Faughnan ME, Kim H, Pourmohamad T, Krings T, Bayrak-Toydemir P, et al. Brain arteriovenous malformation multiplicity predicts the diagnosis of hereditary hemorrhagic telangiectasia: quantitative assessment. Stroke. 2012;43(1):72–8.
Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol. 1990;95(4):422–7.
Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 2010;24(6):203–19.
Matsubara S, Mandzia JL, ter Brugge K, Willinsky RA, Faughnan ME, Manzia JL. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. AJNR Am J Neuroradiol. 2000;21(6):1016–20.
Maher CO, Piepgras DG, Brown Jr RD, Friedman JA, Pollock BE. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32(4):877–82.
Kim H, Marchuk DA, Pawlikowska L, Chen Y, Su H, Yang GY, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206.
Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–72.
Scott BB, McGillicuddy JE, Seeger JF, Kindt GW, Giannotta SL. Vascular dynamics of an experimental cerebral arteriovenous shunt in the primate. Surg Neurol. 1978;10(1):34–8.
Bederson JB, Wiestler OD, Brustle O, Roth P, Frick R, Yasargil MG. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery. 1991;29(3):341–50.
Chaloupka JC, Vinuela F, Robert J, Duckwiler GR. An in vivo arteriovenous malformation model in swine: preliminary feasibility and natural history study. AJNR Am J Neuroradiol. 1994;15(5):945–50.
De Salles AAF, Solberg TD, Mischel P, Massoud TF, Plasencia A, Goetsch S, et al. Arteriovenous malformation animal model for radiosurgery: the rete mirabile. AJNR Am J Neuroradiol. 1996;17(8):1451–8.
Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83(3):539–45.
Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87(2):267–74.
Morgan MK, Anderson RE, Sundt Jr TM. A model of the pathophysiology of cerebral arteriovenous malformations by a carotid-jugular fistula in the rat. Brain Res. 1989;496(1–2):241–50.
Kutluk K, Schumacher M, Mironov A. The role of sinus thrombosis in occipital dural arteriovenous malformations—development and spontaneous closure. Neurochirurgia (Stuttg). 1991;34(5):144–7.
Terada T, Higashida RT, Halbach VV, Dowd CF, Tsuura M, Komai N, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80(5):884–9.
TerBrugge KG, Lasjaunias P, Hallacq P. Experimental models in interventional neuroradiology. AJNR Am J Neuroradiol. 1991;12(6):1029–33.
Kailasnath P, Chaloupka JC. Mathematical modeling of AVM physiology using compartmental network analysis: theoretical considerations and preliminary in vivo validation using a previously developed animal model. Neurol Res. 1996;18(4):361–6.
Massoud TF, Ji C, Vinuela F, Turjman F, Guglielmi G, Duckwiler GR, et al. Laboratory simulations and training in endovascular embolotherapy with a swine arteriovenous malformation model. AJNR Am J Neuroradiol. 1996;17(2):271–9.
Massoud TF, Ji C, Vinuela F, Guglielmi G, Robert J, Duckwiler GR, et al. An experimental arteriovenous malformation model in swine: anatomic basis and construction technique. AJNR Am J Neuroradiol. 1994;15(8):1537–45.
Morgan MK, Anderson RE, Sundt Jr TM. The effects of hyperventilation on cerebral blood flow in the rat with an open and closed carotid-jugular fistula. Neurosurgery. 1989;25(4):606–11.
Murayama Y, Massoud TF, Vinuela F. Hemodynamic changes in arterial feeders and draining veins during embolotherapy of arteriovenous malformations: an experimental study in a swine model. Neurosurgery. 1998;43(1):96–104.
Nagasawa S, Kawanishi M, Kondoh S, Kajimoto S, Yamaguchi K, Ohta T. Hemodynamic simulation study of cerebral arteriovenous malformations. Part 2. Effects of impaired autoregulation and induced hypotension. J Cereb Blood Flow Metab. 1996;16(1):162–9.
Pietila TA, Zabramski JM, Thellier-Janko A, Duveneck K, Bichard WD, Brock M, et al. Animal model for cerebral arteriovenous malformation. Acta Neurochir (Wien). 2000;142(11):1231–40.
Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, et al. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166(6):1883–94.
Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci U S A. 2005;102(28):9884–9.
Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A. 2008;105(31):10901–6.
Su H, Kim H, Pawlikowska L, Kitamura H, Shen F, Cambier S, et al. Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am J Pathol. 2010;176(2):1018–27.
Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, et al. Reducing Jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix Gla protein deficiency. Proc Natl Acad Sci U S A. 2013;110(47):19071–6.
Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS. Vascular morphogenesis: tales of two syndromes. Hum Mol Genet. 2003;12 Suppl 1:R97–112.
Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12(5):473–82.
Bourdeau A, Faughnan ME, Letarte M. Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med. 2000;10(7):279–85.
Satomi J, Mount RJ, Toporsian M, Paterson AD, Wallace MC, Harrison RV, et al. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34(3):783–9.
Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, et al. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab. 2004;24(2):237–44.
Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, et al. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295(6):H2250–6.
Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, et al. VEGF induces more severe cerebrovascular dysplasia in Eng+/− than in Alk1+/− mice. Transl Stroke Res. 2010;1(3):197–201.
Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26(3):328–31.
Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261(1):235–50.
Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119(11):3487–96.
Milton I, Ouyang D, Allen CJ, Yanasak NE, Gossage JR, Alleyne Jr CH, et al. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke. 2012;43(5):1432–5.
Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69(6):954–62.
Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, et al. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke. 2012;43(5):1240–6.
Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol. 2013;33(2):305–10.
Choi EJ, Walker EJ, Shen F, Oh SP, Arthur HM, Young WL, et al. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc Dis. 2012;33(6):540–7.
Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, et al. De novo cerebrovascular malformation in the adult mouse after endothelial alk1 deletion and angiogenic stimulation. Stroke. 2014;45(3):900–2.
Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One. 2014;9(2):e88511.
Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104(10):1343–51.
Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–7.
Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217(1):42–53.
Chen W, Guo Y, Bollen AW, Su H, Young WL. Reduced PDGFR-beta expression after regional Alk1 deletion and VEGF stimulation in the brain is associated with reduced mural cell coverage [Abstract]. Stroke. 2012;43(2-Meeting Abstracts):A3169.
Chen W, Guo Y, Jun K, Wankhede M, Su H, Young WL. Alk1 deficiency impairs mural cell recruitment during brain angiogenesis [Abstract]. Stroke. 2013:44(2-Meeting Abstracts):ATMP118.
Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56(5):1058–65.
Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly Jr T, Stewart CL, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54(2):410–23.
Hasan DM, Amans M, Tihan T, Hess C, Guo Y, Cha S, et al. Ferumoxytol-enhanced MRI to image inflammation within human brain arteriovenous malformations: a pilot investigation. Transl Stroke Res. 2012;3(1):166–73.
Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, et al. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106(8):1425–33.
Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, et al. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156(3):911–23.
Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009;41(1):118–24.
Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCM): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18(5):919–30.
Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93(7):682–9.
Panchenko MP, Williams MC, Brody JS, Yu Q. Type I receptor serine-threonine kinase preferentially expressed in pulmonary blood vessels. Am J Physiol. 1996;270(4 Pt 1):L547–58.
Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62(6):1340–9.
Torsney E, Charlton R, Diamond AG, Burn J, Soames JV, Arthur HM. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation. 2003;107(12):1653–7.
Choi EJ, Walker EJ, Degos V, Jun K, Kuo R, Su H, et al. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke. 2013;44(3):795–8.
Hao Q, Su H, Palmer D, Sun B, Gao P, Yang GY, et al. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke. 2011;42(2):453–8.
van Laake LW, van den Driesche S, Post S, Feijen A, Jansen MA, Driessens MH, et al. Endoglin has a crucial role in blood cell-mediated vascular repair. Circulation. 2006;114(21):2288–97.
Li Y, Hiroi Y, Ngoy S, Okamoto R, Noma K, Wang CY, et al. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123(8):866–76.
Post S, Smits AM, van den Broek AJ, Sluijter JP, Hoefer IE, Janssen BJ, et al. Impaired recruitment of HHT-1 mononuclear cells to the ischaemic heart is due to an altered CXCR4/CD26 balance. Cardiovasc Res. 2010;85(3):494–502.
Tang XN, Zheng Z, Yenari MA. Bone marrow chimeras in the study of experimental stroke. Transl Stroke Res. 2012;3(3):341–7.
Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–40.
Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118(12):3436–9.
Rafii S, Lyden D. Cancer. A few to flip the angiogenic switch. Science. 2008;319(5860):163–4.
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–8.
Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, et al. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28(12):2151–7.
Gao P, Chen Y, Lawton MT, Barbaro NM, Yang GY, Su H, et al. Evidence of endothelial progenitor cells in the human brain and spinal cord arteriovenous malformations. Neurosurgery. 2010;67(4):1029–35.
Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, et al. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27(11):1853–60.
Hashimoto T, Matsumoto M, Tsang EJ, Young WL. Critical roles of neutrophils and macrophages in flow-induced adaptive outward vascular remodeling [Abstract]. J Neurosurg Anesthesiol. 2006;18(4):293.
Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35(10):2294–300.
Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, et al. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–8.
Shenkar R, Shi C, Check IJ, Lipton HL, Awad IA. Concepts and hypotheses: inflammatory hypothesis in the pathogenesis of cerebral cavernous malformations. Neurosurgery. 2007;61(4):693–702.
Aziz MM, Takagi Y, Hashimoto N, Miyamoto S. Activation of nuclear factor κB in cerebral arteriovenous malformations. Neurosurgery. 2010;67(6):1669–80.
Sturiale CL, Puca A, Sebastiani P, Gatto I, Albanese A, Di Rocco C, et al. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: where do we stand? Brain. 2013;136(Pt 2):665–81.
Nagase H, Meng Q, Malinovskii V, Huang W, Chung L, Bode W, et al. Engineering of selective TIMPs. Ann N Y Acad Sci. 1999;878:1–11.
Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34(4):925–31.
Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–91.
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9.
Velasco S, Alvarez-Munoz P, Pericacho M, Dijke PT, Bernabeu C, Lopez-Novoa JM, et al. L- and S-endoglin differentially modulate TGFbeta1 signaling mediated by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci. 2008;121(Pt 6):913–9.
Chen Y, Hao Q, Kim H, Su H, Letarte M, Karumanchi SA, et al. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol. 2009;66(1):19–27.
Velasco-Loyden G, Arribas J, Lopez-Casillas F. The shedding of betaglycan is regulated by pervanadate and mediated by membrane type matrix metalloprotease-1. J Biol Chem. 2004;279(9):7721–33.
Hashimoto T, Matsumoto M, Li JF, Lawton MT, Young WL. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC Neurol. 2005;5(1):1.
Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, et al. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59(1):72–80.
Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115(13):1789–97.
Hosaka K, Hoh BL. Inflammation and cerebral aneurysms. Transl Stroke Res. 2014;5(2):190–8.
Chalouhi N, Jabbour P, Magnotta V, Hasan D. Molecular imaging of cerebrovascular lesions. Transl Stroke Res. 2014;5(2):260–8.
Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45(5):1137–46.
Starke RM, Raper DM, Ding D, Chalouhi N, Owens GK, Hasan DM, et al. Tumor necrosis factor-α modulates cerebral aneurysm formation and rupture. Transl Stroke Res. 2013;5(2):269–77.
Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112(2):232–40.
Thompson S, Kim L, Scott A. Screening for abdominal aortic aneurysm: screening reduces deaths related to aneurysm. BMJ. 2005;330(7491):601.
Achrol AS, Kim H, Pawlikowska L, Poon KY, Ko NU, McCulloch CE, et al. Association of tumor necrosis factor-alpha-238G > A and apolipoprotein E2 polymorphisms with intracranial hemorrhage after brain arteriovenous malformation treatment. Neurosurgery. 2007;61(4):731–9.
Young WL, Kader A, Pile-Spellman J, Ornstein E, Stein BM. Columbia University AVM Study Project. Arteriovenous malformation draining vein physiology and determinants of transnidal pressure gradients. Neurosurgery. 1994;35(3):389–95.
Zhu Y, Lawton MT, Du R, Shwe Y, Chen Y, Shen F, et al. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 2006;59(3):687–96.
Gao P, Zhu Y, Ling F, Shen F, Lee B, Gabriel RA, et al. Nonischemic cerebral venous hypertension promotes a pro-angiogenic state through HIF-1 downstream genes and leukocyte-derived MMP-9. J Cereb Blood Flow Metab. 2009;29(8):1482–90.
Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, et al. Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res. 2004;94(9):1179–85.
Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20(17):4639–47.
Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9(5):707–13.
Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A. 1994;91(11):4678–82.
Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31(1 Pt 2):162–9.
Malek AM, Gibbons GH, Dzau VJ, Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest. 1993;92(4):2013–21.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65(4):476–83.
Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92.
Cirulli A, Liso A, D'Ovidio F, Mestice A, Pasculli G, Gallitelli M, et al. Vascular endothelial growth factor serum levels are elevated in patients with hereditary hemorrhagic telangiectasia. Acta Haematol. 2003;110(1):29–32.
Sadick H, Naim R, Sadick M, Hormann K, Riedel F. Plasma level and tissue expression of angiogenic factors in patients with hereditary hemorrhagic telangiectasia. Int J Mol Med. 2005;15(4):591–6.
Sadick H, Riedel F, Naim R, Goessler U, Hormann K, Hafner M, et al. Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-beta1 as well as high ALK1 tissue expression. Haematologica. 2005;90(6):818–28.
Dupuis-Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E, et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307(9):948–55.
Whitehead KJ, Chakinala M, Faughnan ME, Miller FJ, White Jr RI, Vethanayagam D, et al. Use of Bevacizumab in complicated HHT: North American HHT Center experience [Abstract]. Hematol Rep. 2011;3(s2):6.
Karnezis TT, Davidson TM. Efficacy of intranasal bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope. 2011;121(3):636–8.
Chen S, Karnezis T, Davidson TM. Safety of intranasal Bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope. 2011;121(3):644–6.
Rohrmeier C, Sachs HG, Kuehnel TS. A retrospective analysis of low dose, intranasal injected bevacizumab (Avastin) in hereditary haemorrhagic telangiectasia. Eur Arch Otorhinolaryngol. 2012;269(2):531–6.
Bose P, Holter JL, Selby GB. Bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;360(20):2143–4.
Oosting S, Nagengast W, de Vries E. More on bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;361(9):931.
Retornaz F, Rinaldi Y, Duvoux C. More on bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;361(9):931.
Simonds J, Miller F, Mandel J, Davidson TM. The effect of bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope. 2009;119(5):988–92.
Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, et al. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke. 2012;43(7):1925–30.
Tanvetyanon T, Murtagh R, Bepler G. Rupture of a cerebral arteriovenous malformation in a patient treated with bevacizumab. J Thorac Oncol. 2009;4(2):268–9.
Lukason M, Dufresne E, Rubin H, Pechan P, Li Q, Kim I, et al. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol Ther. 2011;19(2):260–5.
Maclachlan TK, Lukason M, Collins M, Munger R, Isenberger E, Rogers C, et al. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther. 2011;19(2):326–34.
Mao L, Shen F, Chen W, Akinpelu B, Lawton MT, Young WL, et al. AAV-sFLT: a potential therapeutic agent for the treatment of brain angiogenic diseases [Abstract]. Stroke 2013;44(2-Meeting Abstracts):ATMP116.
Frenzel T, Lee CZ, Kim H, Quinnine NJ, Hashimoto T, Lawton MT, et al. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis. 2008;25(1–2):157–63.
Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16(4):420–8.
Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363(9423):1802–11.
Quach H, Kalff A, Spencer A. Lenalidomide in multiple myeloma: Current status and future potential. Am J Hematol. 2012;87(12):1089–95.
D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082–5.
Aragon-Ching JB, Li H, Gardner ER, Figg WD. Thalidomide analogues as anticancer drugs. Recent Pat Anticancer Drug Discov. 2007;2(2):167–74.
Dredge K, Marriott JB, Macdonald CD, Man HW, Chen R, Muller GW, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87(10):1166–72.
Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1–2):56–63.
Sure U, Battenberg E, Dempfle A, Tirakotai W, Bien S, Bertalanffy H. Hypoxia-inducible factor and vascular endothelial growth factor are expressed more frequently in embolized than in nonembolized cerebral arteriovenous malformations. Neurosurgery. 2004;55(3):663–9.
Lim M, Haddix T, Harsh GR, Vogel H, Steinberg GK, Guccione S. Characterization of the integrin alphavbeta3 in arteriovenous malformations and cavernous malformations. Cerebrovasc Dis. 2005;20(1):23–7.
Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, et al. TNFa-238G > A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations [Abstract]. Stroke. 2006;37(2):638–9.
Kim H, Hysi PG, Pawlikowska L, Poon A, Burchard EG, Zaroff JG, et al. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis. 2009;27(2):176–82.
Kiaei M, Petri S, Kipiani K, Gardian G, Choi DK, Chen J, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci Meth. 2006;26(9):2467–73.
Neymotin A, Petri S, Calingasan NY, Wille E, Schafer P, Stewart C, et al. Lenalidomide (Revlimid) administration at symptom onset is neuroprotective in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;220(1):191–7.
Chen CH, Hsu HH, Hu RH, Lee PH, Ho CM. Long-term therapy with thalidomide in hereditary hemorrhagic Telangiectasia: case report and literature review. J Clin Pharmacol. 2012;52(9):1436–40.
Amanzada A, Toppler GJ, Cameron S, Schworer H, Ramadori G. A case report of a patient with hereditary hemorrhagic telangiectasia treated successively with thalidomide and bevacizumab. Case Rep Oncol. 2010;3(3):463–70.
Penaloza A, Vekemans MC, Lambert C, Hermans C. Deep vein thrombosis induced by thalidomide to control epistaxis secondary to hereditary haemorrhagic telangiectasia. Blood Coagul Fibrinolysis. 2011;22(7):616–8.
Alam MA, Sami S, Babu S. Successful treatment of bleeding gastro-intestinal angiodysplasia in hereditary haemorrhagic telangiectasia with thalidomide. BMJ Case Rep. 2011.
Perez-Encinas M, Rabunal Martinez MJ, Bello Lopez JL. Is thalidomide effective for the treatment of gastrointestinal bleeding in hereditary hemorrhagic telangiectasia? Haematologica. 2002;87(8):ELT34.
Gossage JR, Chamberlain SM, Sridhar S, Kumar A. An interim report of thalidomide for treatment of recurrent angioectasia related gastrointestinal bleeding [Abstract #PC10]. Hematol Meet Rep. 2009;3(4):21.
Georgia Health Sciences University. Thalidomide reduces arteriovenous malformation related gastrointestinal bleeding (TAG). NCT00389935. 2011. http://clinicaltrials.gov/ct2/show/NCT00389935?term=thalidomide+hht&rank=2. Accessed 20 Jun 2012.
IRCCS Policlinico S. Matteo. Efficacy of thalidomide in the treatment of hereditary hemorrhagic telangiectasia (THALI-HHT). NCT01485224. 2011. http://clinicaltrials.gov/ct2/show/NCT01485224?term=thalidomide+hht&rank=1. Accessed 20 Jun 2012.
Bowcock SJ, Patrick HE. Lenalidomide to control gastrointestinal bleeding in hereditary haemorrhagic telangiectasia: potential implications for angiodysplasias? Br J Haematol. 2009;146(2):220–2.
Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96(23):13496–500.
Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33(3):831–6.
Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53(6):731–42.
Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125(Pt 6):1297–308.
Bonelli RM, Heuberger C, Reisecker F. Minocycline for Huntington's disease: an open label study. Neurology. 2003;60(5):883–4.
Sanchez-Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48(6):1393–9.
Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, et al. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31(2):325–42.
Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, et al. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33(12):2858–64.
Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett Jr JW, Kent KC, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: Report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36(1):1–12.
Hashimoto T, Matsumoto M, Yang GY, Lawton MT, Young WL. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations [Abstract]. J Neurosurg Anesthesiol. 2004;16(4):333–4.
Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35(7):1715–9.
Acknowledgments
We thank Voltaire Gungab for assistance with manuscript preparation and members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support. The principal investigator, Hua Su, is supported by grants from the National Institutes of Health (R01 NS027713 and P01 NS044155).
Compliance with Ethics Requirements
This review article does not contain any studies with human or animal subjects. All cited studies describe ethical standards in cited manuscripts.
Conflict of Interest
Wanqiu Chen, Eun-Jung Choi, Cameron M. McDougall, and Hua Su declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wanqiu Chen and Eun-Jung Choi contributed equally to this review.
This article is dedicated in memory of our collaborator and mentor William L. Young, MD, for his seminal contributions to brain AVM research.
Rights and permissions
About this article
Cite this article
Chen, W., Choi, EJ., McDougall, C.M. et al. Brain Arteriovenous Malformation Modeling, Pathogenesis, and Novel Therapeutic Targets. Transl. Stroke Res. 5, 316–329 (2014). https://doi.org/10.1007/s12975-014-0343-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-014-0343-0