Abstract
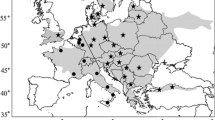
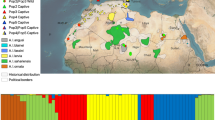
The method presented in here allows for the unequivocal differentiation between non-invasive samples of ocelots and margays, when used in combination with the protocol by Roques et al. (Mol Ecol Resour 11:171–175, 2011). It reduced costs and laboratory processing, decreasing the chances of contamination, and therefore facilitating the identification of large numbers of samples by simple PCR amplification, without the need for sequencing. Our protocol was tested on 371 faeces collected in the field in 25 different sites across the distribution areas of both species, and compared to the mtDNA sequencing of 23 of the samples. The concatenation of a commonly used segment of Cytochrome b with other mitochondrial markers such as ATP-8 allows the identification of these two sister-species, but there was not enough resolution when used alone. With this new method, we could identify 98% of the field faeces, obtaining a global rate of 22.3 faeces of ocelot per margay sample in roads and trails located in potential areas of coexistence, suggesting generally lower densities of margay, although margays could be underestimated due to their arboreal habits. In Serra das Almas, located in the Brazilian Caatinga, the number of faeces of ocelot detected was especially elevated. Low densities of ocelots could be favoring higher densities of margays in areas like the Sinaloan dry forests and the Chimalapas montane forests, both in Mexico. Ocelots were found from open to forested habitats, including mosaic cropland and grassland, however margays were only found in forests where average canopy height exceeded 5 m.



Similar content being viewed by others
References
Bartlett SE, Davidson WS (1992) FINS (forensically informative nucleotide sequencing): a procedure for identifying the animal origin of biological specimens. Biotechniques 12:408–411
Bhavanishankar M, Reddy PA, Gour DS, Shivaji S (2013) Validation of non-invasive genetic identification of two elusive, sympatric, sister-species—tiger (Panthera tigris) and leopard (Panthera pardus). Curr Sci 104:1063–1067
Bianchi RC, Rosa AF, Gatti A, Mendes SL (2011) Diet of margay, Leopardus wiedii, and jaguarundi, Puma yagouaroundi, (Carnivora: Felidae) in Atlantic Rainforest, Brazil. Zoologia 28:127–132
Bontemps S, Defourny P, Van Bogaert E, Arino O, Kalogirou V, Ramos Perez JJ (2011) GLOBCOVER 2009. UCLouvain & ESA Team
Boom R, Sol CJA, Salimans MMM, Jansen CL, Wertheim-van Dillen PME, Van der Noordaa J (1990) Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28:495–503
Calleia FO, Rohe F, Gordo M (2009) Hunting strategy of the Margay (Leopardus wiedii) to attract the wild pied tamarin (Saguinus bicolor). Neotrop Primates 16:32–34
Carvajal-Villarreal S, Caso A, Downey P, Moreno A, Tewes ME, Grassman LI (2012) Spatial patterns of the margay (Leopardus wiedii; Felidae, Carnivora) at “El Cielo” biosphere reserve, Tamaulipas, Mexico. Mammalia 76:237–244
Chaves PB, Graeff VG, Lion MB, Oliveira LR, Eizirik E (2012) DNA barcoding meets molecular scatology: short mtDNA sequences for standardized species assignment of carnivore noninvasive samples. Mol Ecol Resour 12:18–35
Cossíos D, Angers B (2006) Identification of Andean felid species using PCR-RFLP. Mastozool Neotrop 13:239–244
Cuellar E, Maffei L, Arispe R, Noss A (2006) Geoffroy’s cats at the northern limit of their range: activity patterns and density estimates from camera trapping in Bolivian dry forests. Stud Neotrop Fauna Environ 41:169–177
Dalén L, Götherström A, Angerbjörn A (2004) Identifying species from pieces of faeces. Conserv Genet 5:109–111
Davison A, Birks JDS, Brookes RC, Braithwaite TC, Messenger JE (2002) On the origin of faeces: morphological versus molecular methods for surveying rare carnivores from their scats. J Zool 257:141–143
De Oliveira T, Paviolo A, Schipper J, Bianchi R, Payan E, Carvajal S (2015) Leopardus wiedii. The IUCN Red List of Threatened Species 2015: e.T11511A50654216. Accessed 01 Dec 2016
DeMatteo KE et al (2014) Using detection dogs and genetic analyses of scat to expand knowledge and assist felid conservation in Misiones, Argentina. Integr Zool 9:623–639
Di Bitetti MS, De Angelo CD, Di Blanco YE, Paviolo A (2010) Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecol 36:403–412
Donadio E, Buskirk SW (2006) Diet, morphology, and interspecific killing in carnivora. Am Nat 167:524–536
Eizirik E et al (1998) Phylogeographic patterns and evolution of the mitochondrial DNA control region in two neotropical cats (Mammalia, Felidae). J Mol Evol 47:613–624
Emmons LH (1988) A field study of Ocelots (Felis pardalis) in Peru. Rev Ecol Terre Vie 43:133–157
Emmons LH, Feer F (1997) Neotropical rainforest mammals, a field guide, 2nd edn. The University of Chicago Press, Chicago
Endo W (2016) Game Vertebrate Responses to Rural Populations in Neotropical Protected Areas. PhD Thesis, Norwegian University of Life Sciences
Ernest HB, Penedo MCT, May BP, Syvanen M, Boyce WM (2000) Molecular tracking of mountain lions in the Yosemite Valley region in California: genetic analysis using microsatellites and faecal DNA. Mol Ecol 9:433–441
Farrell LE, Roman J, Sunquist ME (2000) Dietary separation of sympatric carnivores identified by molecular analysis of scats. Mol Ecol 9:1583–1590
Fernandes CA, Ginja C, Pereira I, Tenreiro R, Bruford MW, Santos-Reis M (2008) Species-specific mitochondrial DNA markers for identification of non-invasive samples from sympatric carnivores in the Iberian Peninsula. Conserv Genet 9:681–690
Frantz AC, Pope LC, Carpenter PJ, Roper TJ, Wilson GJ, Delahay RJ, Burke T (2003) Reliable microsatellite genotyping of the Eurasian badger (Meles meles) using faecal DNA. Mol Ecol 12:1649–1661
Fraser AF (2012) Feline behaviour and welfare. CABI, Wallingford, pp 84–92
García-Alaníz N, Naranjo EJ, Mallory FF (2010) Hair-Snares: a non-invasive method for monitoring felid populations in the Selva Lacandona, Mexico. Trop Conserv Sci 3:403–411
Goldman EA (1920) Mammals of Panama (with thirty-nine plates). Smithson Misc Collect 96:167–169
Guggisberg CAW (1975) Wild cats of the world. Taplinger Publishing, New York
Haag T et al (2009) Development and testing of an optimized method for DNA-based identification of jaguar (Panthera onca) and puma (Puma concolor) faecal samples for use in ecological and genetic studies. Genetica 136:505–512
Hodge AM (2014) Habitat selection of the margay (Leopardus wiedii) in the eastern Andean foothills of Ecuador. Mammalia 78:351–358
Höss M, Pääbo S (1993) DNA extraction from Pleistocene bones by a silica-based purification method. Nucleic Acids Res 21:3913–3914
Johnson WE, Culver M, Iriarte JA, Eizirik E, Seymour KL, O’Brien SJ (1998) Tracking the evolution of the elusive Andean mountain cat (Oreailurus jacobita) from mitochondrial DNA. J Hered 89:227–232
Johnson WE et al (1999) Disparate phylogeographic patterns of molecular genetic variation in four closely related South American small cat species. Mol Ecol 8:S79-S94
Kasper CB, Schneider A, Oliveira TG (2016) Home range and density of three sympatric felids in the Southern Atlantic Forest, Brazil. Braz J Biol 76:228–232
Kohn MH, Wayne RK (1997) Facts from faeces revisited. Trends Ecol Evol 12:223–227
Konecny MJ (1989) Movement patterns and food habits of four sympatric carnivore species in Belize, Central America. In: Eisenberg K.H. R JF (eds) Advances in neotropical mammology. The Sandhill Crane Press, Gainesville, pp 243–264,
Leopold AS (1959) Wildlife of Mexico. University of California Press, Berkeley, pp 470–475
Li G, Davis BW, Eizirik E, Murphy WJ (2016) Phylogenomic evidence for ancient hybridization in the genomes of living cats (Felidae). Genome Res 26:1–11
Long RA, Mackay P, Zielinski WJ, Ray JC (2008) Non invasive survey methods for carnivores. Island Press, Washington
Longmire JL, Maltbie M, Baker RJ (1997) Use of “lysis buffer” in DNA isolation and its implications for museum collections. Occas Papers Mus Texas Tech Univ 163
Ludlow ME, Sunquist ME (1987) Ecology and behavior of Ocelots in Venezuela. Natl Geogr Res 3:447–461
Martínez-Gutiérrez PG, Palomares F, Fernández N (2015) Predator identification methods in diet studies: uncertain assignment produces biased results? Ecography 38:922–929
Massara RL, Paschoal AMdO, Doherty PF Jr, Hirsch A, Chiarello AG (2015) Ocelot population status in protected Brazilian atlantic forest. PLoS ONE 10:e0141333
Masuda R, Lopez JV, Slattery JP, Yuhki N, O’Brien SJ (1996) Molecular phylogeny of mitochondrial cytochrome b and 12S rRNA sequences in the Felidae: ocelot and domestic cat lineages. Mol Phylogenet Evol 6:351–365
Miotto RA, Rodrigues FP, Ciocheti G, Galetti PM (2007) Determination of the minimum population size of pumas (Puma concolor) through fecal DNA analysis in two protected cerrado areas in the Brazilian Southeast. Biotropica 39:647–654
Morato RG, Ferraz KMPMdB, de Paula RC, Campos CBd (2014) Identification of priority conservation areas and potential corridors for jaguars in the caatinga biome, Brazil. PLoS ONE 9:e92950
Mukherjee S, CN A, Home C, Ramakrishnan U (2010) An evaluation of the PCR-RFLP technique to aid molecular-based monitoring of felids and canids in India. BMC Res Notes 3:159
O’Brien SJ, Johnson WE (2005) Big cat genomics. Annu Rev Genom Hum Genet 6:407–429
Oliveira TG (1994) Neotropical cats: ecology and conservation. Edufma, São Luis
Oliveira TG (1998) Leopardus wiedii. Mamm Species 579:1–6
Oliveira TG (2011) Ecologia e conservação de pequenos felinos no Brasil e suas implicações para o manejo. PhD Thesis, Universidade Federal de Minas Gerais
Oliveira TG et al (2010a) Ocelot ecology and its effect in the small-felid guild in the lowland Neotropics. In: Macdonald DW, Loveridge A (eds) Biology and conservation of wild felids. Oxford University Press, Oxford, pp 563–584
Oliveira R, Castro D, Godinho R, Luikart G, Alves PC (2010b) Species identification using a small nuclear gene fragment: application to sympatric wild carnivores from South-western Europe. Conserv Genet 11:1023–1032
Olson DM, Dinerstein E (1998) The Global 200: a representation approach to conserving the earth’s most biologically valuable ecoregions. Conserv Biol 12:502–515
Palomares F, Adrados B (2014) The use of molecular tools in ecological studies of mammalian carnivores. In: Verdade LM, Lyra-Jorge MC, Piña CI (eds) Applied ecology and human dimensions in biological conservation. Springer, Berlin, pp 105–116
Palomares F, Caro TM (1999) Interspecific killing among mammalian carnivores. Am Nat 153:492–508
Palomares F, Godoy JA, Piriz A, O’Brien SJ, Johnson WE (2002) Faecal genetic analysis to determine the presence and distribution of elusive carnivores: design and feasibility for the Iberian lynx. Mol Ecol 11:2171–2182
Palomares F et al (2012) High proportion of male faeces in jaguar populations. PLoS ONE 7:e52923
Palomares F, Fernández N, Roques S, Chávez C, Silveira L, Keller C, Adrados B (2016) Fine-scale habitat segregation between two ecologically similar top predators. PLoS ONE 11:e0155626
Paviolo A et al. (2016) Leopardus pardalis. The IUCN Red List of Threatened Species 2016: e.T11509A97212355. Accessed 01 December 2016
Paxinos E, McIntosh C, Ralls K, Fleischer R (1997) A noninvasive method for distinguishing among canid species: amplification and enzyme restriction of DNA from dung. Mol Ecol 6:483–486
Payán E (2009) Hunting sustainability, species richness and carnivore conservation in Colombian Amazonia. PhD Thesis, University College London
Prugh LR, Ritland CE (2005) Molecular testing of observer identification of carnivore feces in the field. Wildl Soc Bull 33:189–194
Ramón-Laca A, Soriano L, Gleeson D, Godoy JA (2015) A simple and effective method for obtaining mammal DNA from faeces. Wildl Biol 21:195–203
Rocha-Mendes F, Bianconi GV (2009) Opportunistic predatory behavior of margay, Leopardus wiedii (Schinz, 1821), in Brazil. Mammalia 73:151–152
Rodgers TW, Giacalone J, Heske EJ, Janečka JE, Phillips CA, Schooley RL (2014) Comparison of noninvasive genetics and camera trapping for estimating population density of ocelots (Leopardus Pardalis) on Barro Colorado Island, Panama. Trop Conserv Sci 7:690–705
Roques S, Adrados B, Chavez C, Keller C, Magnusson WE, Palomares F, Godoy JA (2011) Identification of neotropical felid faeces using RCP-PCR. Mol Ecol Resour 11:171–175
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schwartz MK, Luikart G, Waples RS (2007) Genetic monitoring as a promising tool for conservation and management. Trends Ecol Evol 22:25–33
Slattery JP, Johnson WE, Goldman D, O’Brien SJ (1994) Phylogenetic reconstruction of South-American Felids defined by protein electrophoresis. J Mol Evol 39:296–305
Sollmann R, Tôrres NM, Furtado MM, de Almeida Jácomo AT, Palomares F, Roques S, Silveira L (2013) Combining camera-trapping and noninvasive genetic data in a spatial capture–recapture framework improves density estimates for the jaguar. Biol Conserv 167:242–247
Sunquist M, Sunquist F (2002) Wild cats of the world. University of Chicago Press, Chicago
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tortato MA, Oliveira TG, Almeida LB, Beisiegel BM (2013) Valiação do risco de extinção do gato-maracajá Leopardus wiedii (Schinz, 1821) no Brasil. Biodivers Bras 5:76–83
Trigo TC et al (2008) Inter-species hybridization among neotropical cats of the genus Leopardus, and evidence for an introgressive hybrid zone between L. geoffroyi and L. tigrinus in southern Brazil. Mol Ecol 17:4317–4333
Trigo TC, Schneider A, de Oliveira Tadeu G, Lehugeur Livia M, Silveira L, Freitas Thales RO, Eizirik E (2013) Molecular data reveal complex hybridization and a cryptic species of neotropical wild Cat. Curr Biol 23:2528–2533
Trigo TC, Tirelli FP, de Freitas TRO, Eizirik E (2014) comparative assessment of genetic and morphological variation at an extensive hybrid zone between two wild cats in southern Brazil. PLoS ONE 9:e108469
Waits LP, Paetkau D (2005) Non-invasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. J Wildl Manag 69:1419–1433
Wang E (2002) Diets of ocelots (Leopardus pardalis), margays (L. wiedii), and oncillas (L. tigrinus) in the Atlantic rainforest in southern Brazil. Stud Neotrop Fauna Environ 37:207–212
Whitworth A, Braunholtz LD, Huarcaya RP, Beirne C (2016) Out on a limb: arboreal camera traps as an emerging methodology for inventorying elusive rainforest mammals. Trop Conserv Sci 9:675–698
Wultsch C, Waits LP, Kelly MJ (2014) Noninvasive individual and species identification of jaguars (Panthera onca), pumas (Puma concolor) and ocelots (Leopardus pardalis) in Belize, Central America using cross-species microsatellites and faecal DNA. Mol Ecol Resour 14:1171–1182
Acknowledgements
This study was carried out under project BIOCON 05–100/06 of Fundación BBVA, Brazil/Spain joint Project CNPq # 690085/02–8/CSIC # 2004BR0009, Project CGL2010-16902 of the Spanish Ministry of Research and Innovation, Project CGL2013-46026-P of MINECO, and the excellence Project RNM 2300 of Junta de Andalucía. MZ is supported by CNPq DCR fellowship Number 312627/2015-7. Samplings in Brazil were carried out under licenses # 131/2005 CGFAU/LIC of the Brazilian Institute for the Environment and Renewable Natural Resources—IBAMA, and# 11214-1, 13781-1, 13883-1 and 15664-1 SISBIO/Chico Mendes Institute for Biodiversity Conservation—ICMBio, in Mexico under licence # SGPA/DGVS/549 of Dirección General de Vida Silvestre (Semarnat) and in Bolivia under permission from the Sernap (Servicio Nacional de Areas Protegidas) and the DGBAP (Dirección General de Biodiversidad y Areas Protegidas). Faecal and blood samples were exported from Brazil to Spain for genetic analyses under IBAMA/CGEN authorization of access licence # 063/05 and IBAMA/CITES export licences # 0123242BR, 08BR002056/DF and 09BR003006/DF, from Mexico to Spain under export licences # MX33790 and MX42916 of Secretaría de Medio Ambiente/CITES and from Bolivia to Spain with an express permission from the MMayA (Ministerio de Medio Ambiente y Agua). We thank Cap. Ferreira and Lt. Carlos Palhari from the Veterinary Division of the Jungle Warfare Instruction Center of the Brazilian Armed Forces—CIGS, and Diogo Lagroteria and Carlos Abrahão, from IBAMA’s Wildlife Center—NUFAS, in Manaus (Amazonas, Brazil) for facilitating faeces and blood samples from captive felids in their facilities. Raphael de Almeida, Eduardo Ramos, Grasiela Porfírio, Tiago Boscarato, Javier Calzada, Miguel Delibes, Eloy Revilla, Julia Martínez, Gloria Clemencia Amaya, José da Silva Lópes, José Tavares, Carolina Jorge dos Santos, Xênya Garcia Bernardes da Luz, Denise Mello do Prado, Patricia Carignano Torres, Daniel Pererira Munari, Juan Carlos Faller, Meredic Calleja, Erik Joaquín Torres-Romero, Eduardo Espinoza-Medinilla, Ana Alicia Morales and Ricardo Barberí helped with the collection of samples, and Gerardo Ceballos and Heliot Zarza provided logistic support. We thank the managers of the Caiman Ecological Refuge, Serra da Capivara National Park, the Fundação Museu do Homem Americano, the Virua National Park (Bea Lisboa and Antonio Lisboa), Uatumã Biological Reserve, Maracá Ecological Station and the Serra das Almas Natural Reserve (Thiago Roberto Soares Vieira) in Brazil, the Edén Ecological Reserve (Marco Lazcano), El Zapotal Ecological Reserve (Pronatura Península de Yucatán, Juan Carlos Faller and María Andrade) and the Calakmul National Park in Mexico, and AMNI San Matías in Bolivia for their logistical support. Logistical support was provided by Laboratorio de Ecología Molecular, Estación Biológica de Doñana, CSIC (LEM-EBD). Séverine Roques, Noa González and Alberto García assisted in the analysis of samples. Two anonymous referees improved with their comments an earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adrados, B., Zanin, M., Silveira, L. et al. Non-invasive genetic identification of two sympatric sister-species: ocelot (Leopardus pardalis) and margay (L. wiedii) in different biomes. Conservation Genet Resour 11, 203–217 (2019). https://doi.org/10.1007/s12686-018-0992-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12686-018-0992-5