Abstract
The aoudad (Ammotragus lervia) is a Vulnerable bovid endemic of North Africa. Although legally protected in almost every country of its native distribution, the aoudad continues to be hunted for meat and trophy in both North Africa and the countries where it has been introduced. The species was subject to past translocations planned irrespectively of the genetic diversity and local adaptations of source and receiving populations, and no management or conservation units have ever been designed. We aim to provide here important insights on the taxonomy of the aoudad subspecies and the genetic diversity of most of its wild and captive populations. We collected 127 invasive and non-invasive samples from five of the six subspecies of aoudad recognized to date. We could successfully retrieve genetic data for 74 samples. Of those, 36 provided both nuclear (11 microsatellites) and mitochondrial (Cyt-b) data, 31 just nuclear data, and six just mitochondrial data. We implement Bayesian approaches to infer the population structure and phylogenetic relationships between the different populations/subspecies and backtrack the maternal lineages of introduced individuals in European populations. Our results support the presence of four genetically different wild populations, corresponding to three distinct mitochondrial lineages plus a fourth group restricted to Egypt identified by the nuclear markers. We also provide genetic evidence on the affiliation of some introduced European populations with respect to the native ones. The genetic diversity instead of variation within all wild populations was low. This might be a consequence of small effective population size and/or high inbreeding degree, probably related to hunting, decline in habitat availability and quality (i.e. overgrazing, and frequent drought), and high inbreeding degree. Our results provide important information for the aoudad conservation, including reintroductions and reinforcement actions of wild populations, and the exchange of individuals among captive stocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last century, the human impact on the planet’s ecosystems and climate is leading wildlife through a sixth massive extinction (Estes et al. 2011). Human-mediated habitat depletion and overexploitation of natural resources (i.e., mining, logging, and overhunting), and climate change, are acting to threaten the persistence of wildlife and humans populations (Caro et al. 2022). Despite these worrying conditions, there is still inadequate or little information available for most species on which to base effective conservation decisions (Hortal et al. 2015). Within-species genetic diversity is one of the basic levels of biodiversity, which contributes to species persistence by providing adaptations to the environmental traits and changes, and by controlling biotic and abiotic variety (Frankham et al. 2010). The importance of genetic diversity in conservation strategies has been largely addressed already (Hoban et al. 2022, and references therein), being also included in the Convention on Biological Diversity targets (https://www.cbd.int/sp/; Hoban et al. 2020), which aims to prevent genetic erosion and safeguard diversity. The identification of intraspecific genetic patterns (or structures) and levels of population connectivity have also an important role in ensuring effective conservation and management of wildlife (Hoban et al. 2020). Such information is particularly relevant for species with fragmented distributions since it allows to design management and conservation units based on the partial reproductive isolation and/or outbreeding depression criterion (Frankham et al. 2017). Still, biodiversity management projects that routinely target the conservation of genetic diversity are few (Holderegger et al. 2019). Such a gap is usually due to financial restrictions, lack of knowledge, or limited accessibility to study areas (Brito et al. 2014, 2018; Holderegger et al. 2019).
North Africa is an example of how remoteness, low socioeconomic conditions, and political instability can limit scientific research and conservation projects (Brito et al. 2018; UNDP 2020; IEP 2022), resulting in knowledge gaps on local biodiversity (Brito and Pleguezuelos 2020). In particular, Saharan mammals are poorly investigated, and their conservation has attracted less attention in comparison to species in other regions (Brito et al. 2014; Brito et al. 2014). This is even more worrying considering that all large mammals historically inhabiting the Sahara Desert have been either locally extinct (e.g., Panthera leo, Lycaon pictus, Oryx dammah) or at the verge of extinction (IUCN 2019). Saharan ungulates are not an exception, ranking as Vulnerable (Ammotragus lervia, Capra nubiana, Gazella cuvieri, G. dorcas), Endangered (G. leptoceros,), Critically Endangered (Addax nasomaculatus, Nanger dama) or Extinct in the wild (Alcelaphus buselaphus buselaphus, Oryx dammah) (IUCN 2019). The range of most Saharan ungulates has drastically decreased (between the 66% and 99%) mostly due to habitat loss, overgrazing, and poaching (Brito et al. 2014; see Fig. S1 in Brito et al. 2018; Abáigar et al. 2019). The current critical status of Saharan ungulates has prompted several reintroduction projects, aiming to restore depleted or locally extinct ungulate populations (Ramzi et al. 2018; Abáigar et al. 2019; Mertes et al. 2019), but the use of genetic data in reintroduction decisions is still limited (Odgen et al. 2020).
The aoudad (Ammotragus lervia) is an endemic Saharan ungulate inhabiting rugged and mountainous areas of North Africa (Fig. 1; Cassinello et al. 2021). Until the mid-twentieth century, the audad was abundant and widespread across the Sahara (Cassinello 1998), but then it suffered a sharp decline in range and population size, ranking currently as a Vulnerable species (VU) with the total population estimated to be between 5000 and 10,000 individuals (Cassinello et al. 2021). Populations are presently highly fragmented and restricted to rocky mountain regions of limited accessibility, where they are butchered for trophy or bushmeat in poaching raids or where they must compete for natural resources with large domestic livestock (Manlius et al. 2003; Brito et al. 2018; Cassinello et al. 2021). Such factors probably have contributed to an increase in the genetic isolation of populations, which likely amplifies the demographic risks derived from excessive genetic drift and inbreeding. Despite the strong indication that this species is rapidly declining across all its original distribution, a lack of information persists about its genetic diversity and population status (Cassinello et al. 2021). Because of its cultural and economic relevance, the aoudad has been imported as a game species and livestock in several European countries, USA, Mexico, and South Africa (Cassinello et al. 2021); there have also been attempts of introducing the species into the wild, but only in Spain a free-ranging introduction was eventually successful (Cassinello 1998). However, some herds from fenced hunting grounds in the southwestern USA escaped, giving rise to free-ranging aoudad populations (Cugnasse and Tomeï 2016; Cassinello et al. 2021; Wright et al. 2022). Captive populations are also present inside its natural range (Algeria, Morocco and Tunisia; Fig. 2). Despite captive and ex-situ populations could represent a potential source of individuals for reinforcement of wild Sahara populations (Garzón-Machado et al. 2012; Pacioni et al. 2019), little is known about the genetic structure and diversity of wild populations (Derouiche et al. 2020), and about the geographic origin of most of the ex-situ captive populations (Cassinello et al. 2021; Stipoljev et al. 2021). In addition, the morphological variability observed in wild populations has been used to describe six subspecies, but this systematic arrangement has never been genetically assessed and there are uncertainties about the taxonomy and distribution of aoudad’s subspecies (Cassinello et al. 2021).
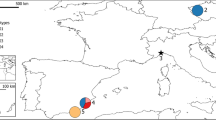
a Distribution of the samples used in the Bayesian clustering analyses. Polygons in the map represent the current estimated distribution of the subspecies (from Cassinello et al. 2021), and the historical. b Boxplot resuming the results of the Bayesian clustering analyses implemented in STRUCTURE program (Evanno et al. 2005). Numbers in the map and STRUCTURE plot correspond to the genetic samples in Table 1 included in this analyse
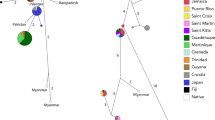
a Distribution of the samples used in the phylogenetic analyses. Polygons in the map represent the current estimated distribution of the subspecies (from Cassinello et al. 2021), and the historical distribution. b Bayesian inferred reconstruction of the evolutionary history of sampled populations. Black dots represent well-supported nodes (PP > 0.95). Colours of numbers represent the clade subdivision of the samples: Clade 1 in green; Clade 2 in red and Clade 3 in yellow. Numbers in the map and phylogenetic tree correspond to the genetic samples in Table 2 included in this analyses
With this study, we aim to answer the following questions: (i) What is the phylogeographic relationship between native and introduced populations in Europe? (ii) How populations are genetically structured in space? (iii) What is the level of genetic diversity of native and introduced populations? (iv) Does the distribution of genetic diversity geographically match the distribution of the current systematic arrangement? Overall, we aim to provide valuable information for the delineation of management and conservation units and for development of management actions targeting the conservation of both wild and captive populations.
Materials and methods
Study area, sampling, and DNA extraction
The study area encompasses the native distribution of aoudad in North Africa, and some Spanish localities where aoudad populations have been introduced (Figs. 1 and 2). We collected 127 samples from 22 different populations distributed in North Africa and Spain (Fig. S1). Collected samples represent five of the six subspecies of aoudad recognized to date. Representative samples for the subspecies angusi, lervia, ornata, and sahariensis were collected from wild individuals in North Africa, while those for the subspecies fassini were collected by wild captured individuals from Libya kept at the zoos of Cordoba (sample NAG1059) and Barcelona (sample NAG1371). Due to the general remoteness of the area and political instability characterizing some countries (Brito et al. 2018), we were not able to obtain samples from the subspecies blainei, and from the populations inhabiting Mauritania, Mali, Chad and Libya. In total, we retrieved 82 samples from wild populations and 45 samples from captive and semi-captive populations (Table S1). Of the samples collected from captive and semi-captive populations, nine were from the Estación Experimental de Zonas Áridas (EEZA; Almeria, Spain), descendants of two wild individuals captured in the Atlantic Sahara; six from the Zoo of Cordoba, Spain (descendants of two individuals from south-east Lybia donated by the Libyan ambassador in Spain); four from the Zoo of Barcelona, Spain (descendants of the stock in the Zoo of Cordoba); six from the Zoo of Madrid, Spain (of unknown origin); three from the zoo of Tunis, Tunisia; and seven from three different semi-captive populations in Tunisia. We also collected samples from a wild population in Sierra Maria-Los Velez mountains (Almería, Spain) originated by vagrant aoudads coming from the nearby mountains of Murcia, where the species was introduced back in 1970 (Cassinello et al. 2004).
Samples included scats, hair, bones, and tissue, and were stored in ethanol (96%) until DNA extraction. Our extraction process followed Maudet et al. (2004), which we adapted with the use of a commercial Kit (E.Z.N.A.® Tissue DNA Kit). We extracted hair and bone samples with a commercial kit (Quiagen® QIAamp DNA Micro Kit). We processed all non-invasive samples (scats, hair, and bones) in dedicated low-quality DNA facilities equipped with positive air pressure and UV lights. We extracted total DNA from tissue samples using the Genomic DNA Minipreps Tissue Kit (EasySpin).
DNA amplification and genetic identification
All genetic identifications relied on the mitochondrial (mtDNA) fragment (450 bp of the Cytochrome-b, Cyt-b) used also for the phylogeographic analysis. Standard protocols for Saharan ungulates were used (Silva et al. 2015). We generated strand sequences using the amplification primers following Tiedemann et al. (2012), allowing the confirmation of sequence consistency and quality. Cycle sequencing reactions were carried out using BIGDYE TERMINATOR v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA). We subsequently separated sequencing products on a 3130xl Genetic Analyser (Applied Biosystems). Sequence alignment was performed using Clustal W (Thompson et al. 1994), and manually checked and reassessed for any discrepancy in BIOEDIT software (Hall 1999). We excluded all the sequences with large amount of missing data.
We amplified eleven nuclear dinucleotide species-transferred microsatellite loci: AMEL, INRA63, OarFCB20, ILST87, INRA172, INRA023, SPS113, MAF065, McM527, INRA005 and INRA006 (all primers recommended for Caprinidae characterization by the International Society of Animal Genetics—ISAG). Forward primers of the 11 markers were fluorescently labelled with VIC, FAM, PET and NED. We arranged the primer pairs into three separate multiplex reactions. PCR amplification was as follows: initial denaturation at 95 °C for 15 min; 30 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 90 s, and elongation at 72 °C for 60 s, and a final elongation at 60 °C for 30 min, according to Glowatzki-Mullis et al. (2007). We conducted a minimum of four replicates polymerase chain reactions, per non-invasive sample per locus to minimize genotyping errors resulting from potential degraded DNA from scats. Fragment analysis was carried out using a 3130xl Genetic Analyser (Applied Biosystems) under standard run conditions with LIZ500 as the internal size standard. We scored alleles and binned using GENEMARKER 1.7.
Population structure and nuclear genetic diversity
GenAlEx 6.5b3 (Peakall et al. 2012) and GENEPOP (Raymond 1995) were used to test for Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium (LD) in the microsatellite data, respectively. Genotyping data were screened with MICRO-CHECKER 2.2.3 (Van Oosterhout et al. 2004) to identify allele dropout, scoring errors due to stuttering, and the presence of null alleles. We also used GenAlEx 6.5b3 to calculate genetic diversity measures, including the number of alleles, effective number of alleles, observed (Ho) and expected (He) heterozygosity, and fixation index (F). We estimated the overall and pairwise genetic differentiation with the θ estimator of Fst (Weir and Cockerham 1984) in FSTAT (Goudad 1995). Statistical significance was determined by 1000 permutations. We used STRUCTURE 2.3.4 (Pritchard et al. 2000) to estimate the nuclear genetic partitioning by inferring the most probable number of genetic clusters. We assumed a number of clusters (K) between one and 20 (i.e., K = 1 to K = 20) for five runs, repeating each run 10 times. We used a burn-in period of 10,000 followed by 1,000,000 Markov chain Monte Carlo (MCMC) steps for each run, choosing the admixture model with the option of correlated allele frequencies. We conducted the same analyses without a priori definition of populations and with sampling locations as prior information (LOCPRIOR option) and lambda parameter was maintained fixed (λ = 1) as default. To verify if the results were not affected by null alleles, we used the recessive alleles option provided in STRUCTURE. We then used Harvester (Earl and VonHoldt 2012) to implement the Evanno method (Evanno et al. 2005) and infer the best-supported clusters.
Phylogeographic reconstruction
We used a Bayesian approach to infer the phylogenetic relationships between the different populations/subspecies and backtrack the maternal lineages of introduced individuals. We aligned the mtDNA sequences with four previously published sequences of the aoudad Cyt-b gene from Derouiche et al. (2020) (GenBank accession numbers MN641980, MN641981, MN641983, and MN641985) using MAFFT v.7 (Katoh et al. 2019) with default parameters (Auto strategy, Gap opening penalty: 1.53, Offset value: 0.0). To root our phylogenetic tree, we used as outgroups published sequences of Arabitragus jayakari (GeneBank access code AY846791.1) and Rupicapra rupicapra (GeneBank access code FJ207539). We deposited all sequences produced for the present study in GenBank. We created a first alignment including 40 sequences of 647 bp (Alignment 1). We also generated a shorter alignment based on 373 bp and including 51 individuals (Alignment 2). We analysed the alignments using BEAST 1.10.4 (Suchard et al. 2018). We determined the best-fit model of sequence evolution using PartitionFinder 2.1.1 (Lanfear et al. 2017) under the Bayesian Information Criterion (BIC). To test whether the genes studied evolve in a clock-like manner (strict clock) we run a preliminary analysis in BEAST 1.10.4 using a relaxed clock. We verified the results of this preliminary run using TRACER 1.6 (http://tree.bio.ed.ac.uk/software/tracer). We rejected the strict clock model when the standard deviation of the uncorrelated lognormal relaxed clock parameter (ucld.stdev) and the coefficient of variation were greater than one. We used a Speciation Yule Process model to assume a constant lineage birth rate for each branch in the tree. We run three separated Markov Chain Monte Carlo (MCMC) analyses for 100 million generations. After a burn-in phase of 10 million generations, trees were sampled every 10,000 generations. We checked chain stationarity and run parameter convergence using TRACER 1.6. Independent runs were combined using LogCombiner v.1.10.0. We assessed tree topologies using TreeAnnotator v.1.10.0 and FigTree v.1.4.4.
Results
From the 127 samples collected for this study (Fig. S1), we could successfully retrieve genetic data for 74 of them. Of those, 36 provided both nuclear and mitochondrial data, 31 just nuclear data, and 6 just mitochondrial data.
Population structure
Our analyses in STRUCTURE suggested the presence of five different clusters (K = 5) as the best ΔK value (results for larger K range (2 < K < 7) can be found in the supplementary material Fig. S2). These clusters match the geographical distribution of our samples, corresponding to populations from: (i) Egypt (Pop1 in brown; Fig. 1); (ii) the Central Sahara, namely from south-east Algeria, Niger and Egypt (Pop2, green; Fig. 1); (iii) the Atlantic Sahara, namely the Atlantic Sahara and southern Morocco (Pop3, red; Fig. 1); (iv) Tunisia (Pop4, yellow; Fig. 1); and (v) Sierra María-Los Vélez in Spain (Pop5, blue; Fig. 1). The individuals kept at the Zoos of Barcelona and Cordoba (Spain) clustered with the Central Sahara populations (Pop2, green; Fig. 1) and the one kept at the Estación Experimental de Zonas Áridas (EEZA, Spain) clustered with the Atlantic Sahara populations (Pop3, red; Fig. 1). Some wild Egyptian individuals (7, 8 and 9 in Fig. 1) displayed affinities from 13% up to 98% with the wild Algeria-Niger population. Signs of admixture were found between populations Pop2 and Pop3 in Niger (individuals 16, 18 and 19; Fig. 1), and in the introduced populations kept at the Zoo of Madrid (Spain) displaying admixed genotypes belonging to Pop3, Pop4, and Pop5.
We did not record stuttering or significant allelic dropout. MICRO-CHECKER suggested the presence of null alleles in populations Pop2 (INRA63, MAF065, OarFCB20, INRA172, and McM527), Pop3 (MAF065 and INRA23), Pop4 (McM527), and Pop5 (INRA06). We found a departure from the Hardy-Weinberg equilibrium for these loci in populations Pop2 for MAF065, OarFCB20, INRA172, and McM527, Pop3 for MAF065, and Pop4 for McM527.
Genetic diversity in wild and captive populations
Given that the Egyptian individuals were identified as a monophyletic group by the mtDNA analyses (although with low support, PP < 93%) and as distinct cluster by the SRUCTURE analyses, we analysed the genetic diversity for the Egyptian population separately from the Algerian and Nigerian ones. The overall FST value was 0.284. The lowest pairwise FST value (FST = 0.179) was found between the Atlantic Sahara (Pop3) and Central Sahara (Pop2) populations, while the highest value (FST = 0.520) was found between the Egyptian (Pop1) and Tunisian (Pop4) populations (Table 2). Global and all pairwise FST values were significantly different from zero (P < 0.01). The eleven microsatellite loci presented a total of 80 alleles, ranging from 2 (Amelogenina) to 11 (INRA23), with a mean number of alleles per locus of 3.61. The Polymorphism Information Contentent (PIC) values ranged from 0.531 to 0.836, with an average of 0.715. We recorded 20 private alleles distributed between the studied populations. We observed the highest average number of private alleles (2.661) in the Atlantic Sahara (Pop3). The observed heterozygosity (Ho) in wild populations ranged from 0.374 (Egyptian, Pop1) to 0.532 (Atlantic Sahara, Pop3). The Ho was always lower than the expected heterozygosity (He), but not for the Atlantic Sahara populations (Ho = 0.532; He = 0.472; x2 = 0.999) (Table 3).
Phylogeographic relationships of native and introduced populations
Partitionfinder 2.1.1 highlighted as best substitution model HKY with gamma model for rate variation. The phylogenetic tree recovered three deep and well supported (posterior probability > 95%) lineages (Fig. 2), corresponding to: (i) Central Sahara lineage including the wild individuals from the mountains of south-eastern Algeria, Niger, and south-western Egypt, some individuals from Sierra María-Los Vélez (Spain), and the captive individuals at the Zoos of Barcelona and Cordoba (Spain) (Clade 1 in green; Fig. 2); (ii) Atlantic Sahara lineage including the wild individuals from the Atlantic Sahara, southern Morocco and north-western Algeria (province of Bechar), some other individuals from Sierra Maria-Los Velez (Spain), and the captive individuals at the Zoo of Madrid and some at EEZA (Spain) (Clade 2 in red; Fig. 2); and (iii) Tunisian lineage including the wild individuals from the mountains of central-west Tunisia, and some other captive individuals at EEZA (Spain), Timimmoun (Algeria), and Chaambi and Boukornine National Parks (Tunisia) (Clade 3 in yellow; Fig. 2).
Nucleotide distance between clades ranged between 0.23% between the Atlantic Sahara lineage (Clade 2) and the Tunisian lineage (Clade 3), and 0.49% between the Egyptian lineage (Clade 1) and the Tunisian lineage (Clade 3) (Table 4).
Implications for taxonomy and systematics
The phylogenetic reconstructions and population structure analyses do not match the current sub-specific taxonomic classification of the aoudad. Our results on the mitochondrial DNA show that: (i) individuals from the subspecies angusi, fassini, ornata and sahariensis cluster in the same monophyletic clade from the Central Sahara Desert distributed from Algeria (province of Bechar), Niger, Chad, Libya, Egypt, and Sudan (Clade 1, in green in Fig. 2); (ii) the clade including the wild individuals inhabiting West Africa, from the Atlantic Sahara and eastern Algeria, is monophyletic and sister to the clade of the subspecies lervia (Clade 2, in red in Fig. 2); (iii) the clade of the subspecies lervia is monophyletic and sister of the above-mentioned clade from Atlantic Sahara, the geographical distribution of the wild populations of lervia clade is restricted to Tunisia (Clade 3, in yellow in Fig. 2). The genetic structure analyses on the nuclear DNA agree with our phylogenetic reconstruction, if not for the Egyptian population forming its own cluster (in brown in Fig. 1).
Discussion
Here we provide the first distribution-wide overview of the mtDNA phylogeography, the nuclear genetic diversity and the genetic structure of the aoudad wild populations. At least three distinct mitochondrial clades were found, and four genetically and geographically distinct populations of aoudad in North Africa were identified. Of these four genetic groups, three were sources of individuals for the introductions in Spain. Additionally, our data do not support the current subspecies classification.
Population structure and signs of gene flow
Our results on the population structure highlighted the presence of four distinct wild populations: Egypt (Pop1), Central Sahara (Algeria, Niger, and Egypt; Pop2), Atlantic Sahara (Pop3), and Tunisia (Pop4). We recorded the highest FST value (FST = 0.520; Table 3) between Egyptian and Tunisian populations, suggesting a long history of divergence and reduced gene flow between the populations living in these areas. On the contrary, signs of admixture were found between the Atlantic and Central Sahara populations (individuals 16, 18, and 19; Fig. 1) and between the Central Sahara and Egyptian populations (individuals 7, 8, and 9; Fig. 1). Likewise, the lowest pairwise FST value (FST = 0.179) was observed between Atlantic and Central Sahara populations. These findings open the hypotheses that gene flow may presently occur between these two populations. The aoudad is known to be able to make small migratory movements in relation to food availability, being capable to survive far from water sources for long periods (even years), relying mostly on water obtained from plants (Cassinello et al. 2021). Natural gene flow may occur between the Atlantic and Central Sahara populations through the Tademait plateau (Algeria), which may potentially connect the populations in the province of Bechar with those in southern regions. Populations in southwestern Egypt have been recorded to conduct long migrations along the Egypt-Libya bordering area (Manlius et al. 2003, and references herein). Local grasslands may ensure ecological connectivity between populations in Egypt and those inhabiting the Tibesti mountains in Chad, which in turn may also be connected with those in Niger. However, individual 4 (Clade 1, in green in Fig. 2, from Algeria), and individual 9 (Pop2, in green in Fig. 1, from Egypt), show different haplotypes and genotypes from the other individuals of the populations where the samples were collected (Egyptian and Nigerian populations, respectively). Game species are often subject to translocations of individuals to re-establish or strengthen overhunted populations (Silva et al. 2015; Cassinello et al. 2021; Stipoljev et al. 2021). For instance, in Naâma (Algeria) thousands of ungulates of captive breeding origins are systematically released since 2016 on the scope of a reintroduction programme of the Algerian government in partnership with the United Arabic Emirates (https://www.djazairess.com/fr/lesoirdalgerie/1019815). These translocations might have been made irrespectively of the genetic diversity and local adaptations of source and receiving populations (Silva et al. 2015; Cassinello et al. 2021; Stipoljev et al. 2021). For this reason, it is unclear if the genetic admixture we recorded is caused by natural gene flow or if it is the result of human-mediated translocation events. Additional sampling and landscape genetics studies are needed to test for the presence and levels of gene flow between these populations, as well as the occurrence of functional population connectivity.
Genetic diversity in wild and captive populations
Despite the relatively limited sample size of our study, our preliminary results highlight the worrisome low genetic diversity of the native populations of this species. We recorded low genetic diversity in both, native and introduced populations (Table 4). Except for the Atlantic Sahara ones, all sampled populations displayed lower observed heterozygosity (Ho) than expected (He). The observed low genetic diversity is likely the result of high levels of inbreeding (Slate et al. 2004), probably caused by the continuous decline in range and population size recorded for the native populations of this species over the last century (Kowalski and Rzebik-Kowalska 1991; Smet 1997; Bounaceur et al. 2016; Derouiche et al. 2020; Cassinello et al. 2021). Reports from scientific expeditions in Algeria, Niger, and Tunisia during the 19th century testify that the aoudad was widely distributed in these regions at the time (e.g. Gervais 1848; Aucapitaine 1856; Loche 1867; Colomb 1858; Tristram 1860). However, a decline caused by poaching and habitat loss started at the beginning of the 20th century (Joleaud 1927), and continued until today, leading the Central Sahara populations on the verge of extinction (Bounaceur et al. 2016; Derouiche et al. 2020; Gašparová et al. 2020). The Egyptian population is also suffering from low genetic diversity, displaying the lowest levels of genetic diversity compared to both African and Spanish populations (Pop1; Ho= 0.304 He= 0.427) (Table 4). As in other areas of its distribution, in less than two centuries the Egyptian aoudad went from being spread all over Egypt (until the Arabian Peninsula) to being present in just three remote locations on the border with Libya and Sudan (Amer 1997). Further genetic assessments of aoudad’s wild populations are needed to validate the current genetic patterns found and deepen the determination of intraspecific genetic diversity of each specific population.
Spanish populations have the highest difference between expected (He= 0.616) and observed (Ho= 0.403) heterozygosity among all the populations analysed. Low levels of mitochondrial and nuclear genetic diversity were already recorded in European populations by Stipoljev et al. (2021). The study also highlighted that the populations from Sierra María-Los Velez might be the ones with the highest genetic diversity among all the populations introduced in Europe, which is in line with our phylogenetic results showing the presence of at least two maternal clades from Atlantic and Central Sahara (red and green in Fig. 2). Overall, our preliminary results suggest that before including individuals from Spain in future reintroduction projects, the genetic diversity of these individuals should be carefully evaluated to avoid interbreeding in wild populations.
Null alleles and allelic dropout are particularly frequent when analysing non-invasive samples, due to the low quality (and quantity) of DNA found in these sample types (Pompanon et al. 2005). The null alleles and Hardy-Weinberg disequilibrium recorded for some of the loci targeted in this study likely derive from the un-specificity of the markers used, originally developed for the genus Ovis (ISAG). In future studies, primers specifically designed for the aoudad should be implemented to counteract for the presence of genotyping errors and for giving more precise information on population genetic structure and diversity of this species.
Phylogeographic relationship of native and introduced populations
Our phylogenetic reconstruction groups the North African aoudad populations in three distinct mitochondrial lineages from the Atlantic Sahara, Tunisia, and Central Sahara. This classification is discordant with the current systematic of the species (discussed below) based on morphological characters (see Cassinello 1998), but sheds light on the likely African origin of the introduced populations in Spain: (i) individuals sampled in the Zoo of Madrid display mitochondrial affinities to the lineage from the Atlantic Sahara (Clade 2 in red; Fig. 2), but admixed genotypes belonging to populations from Tunisia, and Sierra María-Los Velez (Spain); (ii) individuals sampled in the Zoos of Cordoba and Barcelona display similar genotypes and mitochondrial affinities to the Central Sahara populations (Clade 1 and Pop2 in green in Figs. 1 and 2); (iii) individuals at the EEZA research centre in Almeria (Spain) clustered with individuals from the Atlantic Sahara and Tunisia lineages (individual number 42 in Fig. 2); (iv) individuals from the introduced population in Sierra María-Los Velez cluster with individuals from the Atlantic and Central Sahara lineages (specifically with an individual from Niger). The populations kept at the EEZA are known to have originated from a wild couple captured in the Atlantic Sahara, and the population in Sierra María-Los Velez to have originated from individuals from the Frankfurt Zoo in Germany and the Ain Sebad Zoo in Casablanca (Morocco) (Stipoljev et al. 2021). The Frankfurt Zoo and the Ain Sebad Zoo were also the source for other European populations in Croatia (Mosor Mountain), the Czech Republic (surroundings of Plzeň), Murcia and La Palma (Spain) (Stipoljev et al. 2021). Since the individuals kept in the Ain Sebad Zoo likely belong to the Atlantic Sahara lineage (Stipoljev et al. 2021), we hypothesised that at least some individuals kept in the Zoo of Frankfurt belonged to a population in Niger (Central Sahara lineage). However, additional analyses involving genomic data are needed to test the actual origin and genetic adaptations of individuals from captive populations before future reinforcement of wild populations with captive bred individuals can be proposed.
Implications for taxonomy and systematics
In line with our phylogenetic reconstruction and genetic structure analyses from the sampled wild populations we recommend the following systematic subdivision: (i) The subspecies angusi, fassini, ornata, and sahariensis should be considered as a single subspecies, distributed from north-western Algeria to Niger and Egypt; (ii) the Atlantic Sahara population, previously included to the subspecies sahariensis, should be considered as a unique taxonomic entity (potentially a new subspecies) distributed from the Atlantic Sahara region to the province of Bechar (Algeria); and (iii) the distribution of the nominal subspecies lervia should be restricted to Tunisia. The Egyptian individuals grouped together (although with low support; PP = 93; Fig. 2) in the phylogenetic analyses and STRUCTURE bar plot (in brown in Fig. 1) and it presents the highest FST values if compared with the other four populations (Table 3). This line of evidence may instead suggest the validity of the subspecies ornata endemic to Egypt, and previously described based on morphological traits (see Cassinello 1998). However, one individual from Algeria (number 4 in Fig. 2; retrieved from Derouiche et al. 2020) grouped within the Egyptian lineage in the phylogenetic tree. Although Derouiche et al. (2020) collected samples from this individual in the wild, it cannot be excluded that individual comes from a previous translocation of Egyptian individuals to Algeria aimed in restoring the Algerian populations that have been massively hunted in the past (i.e. as it is happening in Naâma; De Smet 1997; Derouiche et al. 2020). For this reason, further genetic and morphological data are needed to corroborate the existence of the subspecies ornata. The current study refrains from formally redefining the systematic of the aoudad and testing validity of the subspecies ornata and of the Atlantic Sahara lineage as new subspecies. These two topics should be addressed in an integrative taxonomy approach (Padial et al. 2010), including additional genetic (samples and markers), ecological, morphological, and distributional data.
Conclusions and conservation recommendations
Despite being protected by national law, the management of aoudad wild populations still needs improvements in most of the species’ distribution. Future translocation projects might be needed to reinforce the native populations, but this may result ineffective if poaching and habitat loss endure (Silva et al. 2015; Gardner et al. 2021). Moreover, translocations are an important conservation tool, but their success depends also on retaining genetic fitness and evolutionary potential (Pacioni et al. 2019). Translocations ignoring the genetic diversity distribution could have deleterious effects on wild populations as diluting the adaptation to climate or pathogen load, leading to high mortality and poor success of the management intervention (Bertola et al. 2022). The data we provide in this study allowed determining the aoudad main genetic units in the native distribution of the species, laying the ground for a more comprehensive genetic analysis of its native populations. In light of the results found, we suggest the following conservation actions: (i) conducting regular census in all known wild populations to keep monitoring demographic trends; (ii) implementing a landscape genomic approach to uncover local genetic adaptations and ecological corridors used by the species; (iii) including samples from individuals of the subspecies blainei to formally redefining the subspecific taxonomic units through an integrative taxonomy approach; (iv) to promote the establishment of international protected areas where hunting is strictly prohibited or carefully regulated; (v) use genomic data to assess the genetic diversity of all European and American introduced populations, to highlight their potential role as source individuals for future reintroductions; (vi) collaborating with local communities to find alternative livelihood project to poaching (i.e. ecotourism); and (vii) reintroduction, translocation, and reinforcement programs should consider at first the design of management units, which delineation should be based on the partial reproductive isolation/outbreeding depression.
Data availability
The datasets generated during and/or analysed during the current study are available as supporting material (Alignments 1 and 2) and in the GenBank.
References
Abáigar T, Rodríguez-Caballero E, Martínez C, Amaouch Z, Samlali ML, Aparicio F, El Balla T, Essalhi A, Fernández J, García F, Haya M (2019) The first reintroduction project for mhorr gazelle (Nanger dama mhorr) into the wild: knowledge and experience gained to support future conservation actions. Glob Ecol Conserv 19:e00680
Amer M (1997) Egypt. In: Shackleton DM (ed) Wild Sheep and goats and their relatives. Status survey and conservation action plan for caprinae. IUCN, Gland, pp 21–26
Ashlock PD, Mayr E (1991) Principles of systematic zoology. McGraw-Hill, Inc., New York. https://doi.org/10.2307/3283335
Aucapitaine H (1856) Notice sur le mouflon à manchettes, ou mouflon d’Afrique. Rev Mag Zool 28:3–5
Aulagnier S, Thévenot M (1997) Morocco (including western Sahara). In: Shackleton DM (ed) Wild sheep and goats and their relatives. Status survey and conservation action plan for caprinae. IUCN, Gland, pp 34–38
Bertola LD, Miller SM, Williams VL, Naude VN, Coals P, Dures SG, Henschel P, Chege M, Sogbohossou EA, Ndiaye A, Kiki M (2022) Genetic guidelines for translocations: maintaining intraspecific diversity in the lion (Panthera leo). Evol Appl 15(1):22–39
Beudels-Jamar RC, Devillers P, Lafontaine RM, Devillers-Tershuren J, Beudels MO (2006) Sahelo-Saharan Antelopes. Status and perspectives. CMS Technical Series Publication No. 11. UNEP/CMS, Bonn
Bounaceur F, Benamor N, Bissaad FZ, Abdi A, Aulagnier S (2016) Is there a future for the last populations of aoudad (Ammotragus lervia) in northern Algeria? Pakistan J Zool 48:1727–1731
Brito JC, Godinho R, Martínez-Freiría F, Pleguezuelos JM, Rebelo H, Santos X, Vale CG, Velo-Antón G, Boratyński Z, Carvalho SB, Ferreira S, Gonçalves DV, Silva TL, Tarroso P, Campos JC, Leite JV, Nogueira J, Álvares F, Sillero N, Sow AS, Fahd S, Crochet P-A, Carranza S (2014) Unravelling biodiversity, evolution and threats to conservation in the Sahara-Sahel. Biol Rev 89:215–231. https://doi.org/10.1111/brv.12049
Brito JC, Durant SM, Pettorelli N, Newby J, Canney S, Algadafi W, Rabeil T, Crochet P-A, Pleguezuelos JM, Wacher T, de Smet K, Gonçalves DV, da Silva MJF, Martínez-Freiría F, Abáigar T, Campos JC, Comizzoli P, Fahd S, Fellous A, Garba HHM, Hamidou D, Harouna A, Hatcha MH, Nagy A, Silva TL, Sow AS, Vale CG, Boratyński Z, Rebelo H, Carvalho SB (2018) Armed conflicts and wildlife decline: challenges and recommendations for effective conservation policy in the Sahara-Sahel. Conserv Lett 11:e12446. https://doi.org/10.1111/conl.12446
Brito JC, Pleguezuelos JM (2020) Desert biodiversity—World’s hot spots/globally outstanding biodiverse deserts. In: Goldstein MI, DellaSala DA (eds) Encyclopedia of the World’s biomes. Deserts—life in the extremes. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-409548-9.11794-4
Brito JC, Sow SA, Vale CG, Pizzigalli C, Hamidou D, Gonçalves DV, Martínez-Freiría F, Santarém F, Rebelo H, Campos JC, Pleguezuelos JM, Ferreira da Silva MJ, Naia M, Tarroso P, Godinho R, Silva TL, Macedo T, Boratyński Z, El Abidine Sidatt Z, Álvares F (2022) Diversity, distribution and conservation of land mammals in Mauritania, North-West Africa. PLoS ONE 17:e0269870. https://doi.org/10.1371/journal.pone.0269870
Caro T, Rowe Z, Berger J, Wholey P, Dobson A (2022) An inconvenient misconception: climate change is not the principal driver of biodiversity loss. Conserv Lett e12868. https://doi.org/10.1111/conl.12868
Cassinello J (1998) Ammotragus lervia: a review on systematics, biology, ecology and distribution. Ann Zool Fenn 35:149–162
Cassinello J, Serrano E, Calabuig G, Pérez JM (2004) Range expansion of an exotic ungulate (Ammotragus lervia) in southern Spain: ecological and conservation concerns. Biodivers Conserv 13:851–866. https://doi.org/10.1023/B:BIOC.0000014461.69034.78
Cassinello J, Bounaceur F, Brito JC, Bussière E, Cuzin F, Gil-Sánchez J, Herrera-Sánchez F, Wacher T (2021) Ammotragus lervia. The IUCN Red List of Threatened Species 2021: e.T1151A22149987
Colomb L (1858) Exploration des Ksours et du Sahara de la Province d’Oran. Imprimerie du Gouvernement, Alger
Cugnasse JM, Tomeï N (2016) Le Mouflon à manchettes Ammotragus lervia (Pallas, 1777). Les Mammifères de Provence-Alpes-Côte d’Azur. Biotope, Mèze. LPO PACA, GECEM & GCP
De Smet K (1997) Algeria. In: Shackleton DM (ed) Wild sheep and goats and their relatives. Status survey and conservation action plan for caprinae. IUCN, Gland, pp 17–19
Derouiche L, Irzagh A, Rahmouni R, Tahri R, Hadjeloum M, Bouhadad R, Fernandes C (2020) Deep mitochondrial DNA phylogeographic divergence in the threatened aoudad Ammotragus lervia (Bovidae, Caprini). Gene 739:144510. https://doi.org/10.1016/j.gene.2020.144510
Earl DA, Von Holdt BM (2012) Structure harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Gen Res 4:359–361
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marques RJ, Oksanen L, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet earth. Science 333:301–306. https://doi.org/10.1126/science.1205106
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Fennessy J, Bidon T, Reuss F, Kumar V, Elkan P, Nilsson MA, Vamberger M, Fritz U, Janke A (2016) Multi-locus analyses reveal four giraffe species instead of one. Curr Biol 26:2543–2549. https://doi.org/10.1016/j.cub.2016.07.036
Frankham R (2010) Challenges and opportunities of genetic approaches to biological conservation. Biol Conserv 143(9):1919–1927. https://doi.org/10.1016/j.biocon.2010.05.011
Frankham R, Ballou JD, Ralls K, Eldridge M, Dudash MR, Fenster CB, Lacy CB, Sunnucks P (2017) Genetic management of fragmented animal and plant populations. Oxford University Press, Oxford. https://doi.org/10.1093/oso/9780198783398.001.0001
Furlan E, Stoklosa J, Griffiths J, Gust N, Ellis R, Huggins RM, Weeks AR (2012) Small population size and extremely low levels of genetic diversity in island populations of the platypus, Ornithorhynchus anatinus. Ecol Evol 2:844–857. https://doi.org/10.1002/ece3.195
Gardner PC, Goossens B, Bakar SBA, Bruford MW (2021) Hunting pressure is a key contributor to the impending extinction of Bornean wild cattle. Endang Spec Res 45:225–235. https://doi.org/10.3354/esr01112
Gašparová K, Fennessy J, Rabeil T, Zabeirou AR, Pourchier C, Brown M, Brandlová K (2020) 3rd Quarterly Report West African giraffe (Giraffa camelopardalis peralta) Republic of Niger
Garzón-Machado V, del-Arco-Aguilar MJ, Pérez-de-Paz PL (2012) Threat or threatened species? a paradox in conservation biology. J Nat Conserv 20:228–230. https://doi.org/10.1016/j.jnc.2012.03.001
Gervais J (1848) Sur les animaux vertébrés de l’Algérie, envisagés sous le double rapport de la géographie zoologique et de la domestication. Ann Sci Nat Zool 3:202–208
Glowatzki-Mullis ML, Muntwyler J, Gaillard C (2007) Cost-effective parentage verification with 17-plex PCR for goats and 19-plex PCR for sheep. Ani Gen 38:86–88. https://doi.org/10.1111/j.1365-2052.2006.01550.x
Godinho R, Abáigar T, Lopes S, Essalhi A, Ouragh L, Cano M, Ferrand N (2012) Conservation genetics of the endangered Dorcas gazelle (Gazella dorcas spp.) in Northwestern Africa. Conserv Gen 13:1003–1015
Gonçalves DV, Martínez-Freiría F, Crochet P-A, Geniez P, Carranza S, Brito JC (2018) The role of climatic cycles and trans-saharan migration corridors in species diversification: biogeography of Psammophis schokari group in North Africa. Mol Phylog Evol 118:64–74. https://doi.org/10.1016/j.ympev.2017.09.009
Goudet J (1995) FSTAT (version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486. https://doi.org/10.1093/oxfordjournals.jhered.a111627
Gray GG, Simpson CD (1980) Ammotragus lervia. Mamm Spec 144:1–7
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuc Acid Symp Ser 41:95–98
Hoban S, Bruford M, Jackson JDU, Lopes-Fernandes M, Heuertz M, Hohenlohe PA, Paz-Vinas V, Sjogren-Gulve P, Segelbacher G, Vernesi C, Aitken S, Bertola LD, Bloomer P, Breed M, Rodriguez-Correra H, Funk WC, Gruber CE, Hunter ME, Jaffe R, Liggins L, Mergeay J, Moharrek F, O’Brien D, Ogden R, Palma-Silva C, Pierson J, Ramakrishan U, Simo-Droissart M, Tani N, Waits L, Laikre L (2020) Genetic diversity targets and indicators in the CBD post-2020 global Biodiversity Framework must be improved. Biol Conserv 248:108654. https://doi.org/10.1016/j.biocon.2020.108654
Hoban S, Archer FI, Bertola LD, Bragg JG, Breed MF, Bruford MW, Coleman MA, Ekblom R, Funk WC, Grueber CE, Hand BK, Jaffé R, Jensen E, Johnson JS, Kershaw F, Liggins L, MacDonald AJ, Mergeay J, Miller JM, Muller-Karger F, O’Brien D, Paz-Vinas I, Potter KM, Razgour O, Vernesi C, Hunter ME (2022) Global genetic diversity status and trends: towards a suite of essential biodiversity variables (EBVs) for genetic composition. Biol Rev. https://doi.org/10.1111/brv.12852
Holderegger R, Balkenhol N, Bolliger J, Engler JO, Gugerli F, Hochkirch A, Nowak C, Segelbacher G, Widmer A, Zachos FE (2019) Conservation genetics: linking science with practice. Mol Ecol 28:3848–3856. https://doi.org/10.1111/mec.15202
Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ (2015) Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol Syst 46(1):523–549. https://doi.org/10.1146/annurev-ecolsys-112414-054400
Hummel J, Steuer P, Südekum K-H, Hammer S, Hammer C, Streich WJ, Clauss M (2008) Fluid and particle retention in the digestive tract of the addax antelope (Addax nasomaculatus) — adaptations of a grazing desert ruminant. Comp Biochem Physiol A149:142–149. https://doi.org/10.1016/j.cbpa.2007.11.001
Institute for Economics & Peace (2022) Global peace index 2022: measuring peace in a complex world, Sydney
IUCN (2019) Summary statistics. Gland, Switzerland and Cambridge, UK: IUCN. [cited from 2022 August 22]. https://www.iucnredlist.org/resources/summary-statistics#Summary%20Tables
Joleaud L (1927) Etudes de géographie zoologique sur la Berbérie. Le Mouflon à manchettes. CR Soc Biogéogr 4:43–45. https://shs.hal.science/halshs-03782908
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20(4):1160–1166
Kazutaka K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. https://doi.org/10.1093/bib/bbx108
Kowalski K, Rzebik-Kowalska B (1991) Mammals of Algeria. Polish Academy of Science, Institute of Systematics and Evolution of Animals. Ossolineum, Wroclaw. https://doi.org/10.2307/1382086
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B (2017) PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol 34:772–773. https://doi.org/10.1093/molbev/msw260
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Liz AV, Rödder D, Gonçalves DV, Velo-Antón G, Fonseca MM, Geniez P, Crochet P-A, Brito JC (2021) The role of Sahara highlands in the diversification and desert colonization of the Bosc’s fringe-toed lizard. J Biogeog 48:2891–2906. https://doi.org/10.1111/jbi.14250
Loche V (1867) Histoire naturelle des mammifèresExploration Scientifique de l’Algérie Pendant les Années. Imprimerie Royale, Paris, p 1842
Loggers CO, Thévenot M, Aulagnier S (1992) Status and distribution of moroccan wild ungulates. Biol Conserv 59:9–18. https://doi.org/10.1016/0006-3207(92)90708-U
Manlius N, Menardi-Noguera A, Zboray A (2003) Decline of the Barbary sheep (Ammotragus lervia) in Egypt during the 20th century: literature review and recent observations. J Zool 259(4):403–409
Maudet C, Luikart G, Dubray D, Von Hardenberg A, Taberlet P (2004) Low genotyping error rates in wild ungulate faeces sampled in winter. Mol Ecol Not 4:772–775. https://doi.org/10.1111/j.1471-8286.2004.00787.x
Mertes K, Stabach JA, Songer M, Wacher T, Newby J, Chuven J, Dhaher SA, Leimgruber P, Monfort S (2019) Management background and release conditions structure post-release movements in reintroduced ungulates. Front Ecol Evol 7:470. https://doi.org/10.3389/fevo.2019.00470
Ogden R, Chuven J, Gilbert T, Hosking C, Gharbi K, Craig M, Salem Al Dhaheri S, Senn H (2020) Benefits and pitfalls of captive conservation genetic management: evaluating diversity in scimitar-horned oryx to support reintroduction planning. Biol Conserv 241:108244. https://doi.org/10.1016/j.biocon.2019.108244
Pacioni C, Wayne AF, Page M (2019) Guidelines for genetic management in mammal translocation programs. Biol Conserv 237:105–113. https://doi.org/10.1016/j.biocon.2019.06.019
Padial JM, Miralles A, De la Riva I, Vences M (2010) The integrative future of taxonomy. Front Zool 7(1):1–4
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Pompanon F, Bonin A, Bellemain E, Tabeler P (2005) Genotyping errors: causes, consequences and solutions. Nat Rev Genet 6:847–859. https://doi.org/10.1038/nrg1707
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959. https://doi.org/10.1093/genetics/155.2.945
Ramzi H, Qarro M, El Abidine AZ, Abrioui A (2018) Conservation d’une population de Mouflon à manchettes (Ammotragus lervia Pallas, 1777) (Mammalia, Bovidae) dans un espace clos: cas de la réserve d’Amassine dans le Haut-Atlas (Parc National du Toubkal, Maroc). Terre et Vie 73:474–491. https://hal.science/hal-03532892
Raymond M (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249. https://doi.org/10.1093/oxfordjournals.jhered.a111573
Silva TL, Godinho R, Castro D, Abáigar T, Brito JC, Alves PC (2015) Genetic identification of endangered north african ungulates using non-invasive sampling. Mol Ecol Resour 15:652–661. https://doi.org/10.1111/1755-0998.12335
Slate J, David P, Dodds KG, Veenvliet BA, Glass BC, Broad TE, McEwan JC (2004) Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity 93:255–265. https://doi.org/10.1038/sj.hdy.6800485
Stipoljev S, Safner T, Gančević P, Galov A, Stuhne T, Svetličić I, Grignolio S, Cassinello J, Šprem N (2021) Population structure and genetic diversity of non-native aoudad populations. Sci Rep 11:1–9. https://doi.org/10.1038/s41598-021-91678-2
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A (2018) Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 4:vey016. https://doi.org/10.1093/ve/vey016
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acid Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Tiedemann R, Cipriano F, Morin PA, Hoelzel AR, Palsbøll P, Waples R, Natoli A, Bachmann L, Postma L, Double M, Pampoulie C, Skaug H, Baker CS, Jackson J (2012) Updated guidelines for DNA data quality control and error rate estimation, for genetic studies relevant to IWC management advice. Report to the Scientific Committee of the International Whaling Commission. Paper SC/64/SD4 presented at the IWC, Panama City
Tristram HB (1860) The Great Sahara: Wanderings south of the Atlas Mountains. John Murray, London
UNDP (2020) Description. xi, 397 p.: graphs, tables. Notes. Includes bibliographical references, pp. 291–332
Van Oosterhout C, Hutchinson WF, Wills DP, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Note 4:535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370. https://doi.org/10.2307/2408641
Woolston C (2016) DNA reveals that giraffes are four species — not one. Nature 537:290–291. https://doi.org/10.1038/nature.2016.20567
Wright EA, Wiedmeier RC, Roberts EK, Pipkin DR, Hernández F, Bayouth JP, Conway WC, Bradley RD (2022) Distinct mtDNA lineages in free-ranging Ammotragus (aoudad) from the United States indicate multiple introductions from northern Africa. Ecol Evol 12(4):e8849. https://doi.org/10.1002/ece3.8849
Zwickl DJ, Hillis DM (2002) Increased taxon sampling greatly reduces phylogenetic error. Syst Biol 51:588–598. https://doi.org/10.1080/10635150290102339
Acknowledgements
We acknowledge J Abdelkader, M Cano, JM Gil-Sanchéz, H Fernández (Barcelona Zoo), S Ferreira, F Martínez-Freiría, K De Smet, M Petretto, F Germi, H Senn, M Jamel, M Medianni, EM Nevado (Madrid Zoo), T Rabeil, AJ Rodríguez, Harmusch Ass, Mª Josefa Ruiz Aguilera, and the Andalusian Agency for the Environment for generously providing samples, and Francisco Valera for granting access to specimens in the ‘La Hoya’ Experimental Farm (EEZA, CSIC), along with J. Benzal and its technical staff. We acknowledge S Mourão and S Lopes for laboratory support.
Funding
Open access funding provided by FCT|FCCN (b-on). This study was partially supported by ECWP – Emirates Center for Wildlife Propagation, FCT-Fundação para a Ciência e Tecnologia in the scope of the project UIDP/50027/2020, and FEDER funds through the Operational Programme for Competitiveness Factors – COMPETE (FCOMP-01-0124-FEDER-008917/028276). JCB and CP are supported by FCT (CEECINST/00014/2018/CP1512/CT0001, 2020.05054.BD). None of the funding entities were involved in the study design, data collection, analysis or interpretation of data, writing or decision to submit this manuscript.
Author information
Authors and Affiliations
Contributions
TA and JCB conceived the ideas; TLS, TA, and JCB collected the data; CP, TLS and GB analysed the data; CP led the writing with inputs from all authors.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval
Sample collection made under authorisation from the Haut-Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification of Morocco (permit 05/2016), and the Direction Générale des Fôrets of Algeria and of Tunisia (in the frame of collaboration conservation projects between the EEZA and this countries). The Sahara Conservation Fund provided support for sample collection in Niger and Egypt. Analyses were performed at the CITES registered laboratory 13PT0065/S. Institutional Animal Care and Use Committee (IACUC) approval was not requested as no animal was sacrificed, no animal husbandry was needed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pizzigalli, C., Silva, T.L., Abáigar, T. et al. Assessment of population structure and genetic diversity of wild and captive populations of Ammotragus lervia provide insights for conservation management. Conserv Genet 25, 59–73 (2024). https://doi.org/10.1007/s10592-023-01546-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-023-01546-x