Abstract
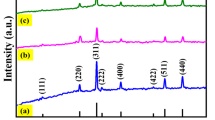
The reduced graphene oxide/manganese ferrite (rGO/MnFe2O4) composite was prepared via a simple and straight forward solvothermal technique. The well-defined and mono-dispersed MnFe2O4 spherical particles with the average particle size of 370 nm, composing of 10-nm-sized primary nanoparticles were densely anchored over MnFe2O4 composite cons support. The cubic spinel structure of MnFe2O4 nanoparticles was unfurled through the diffraction patterns, and its compilation with rGO to form composite has sustained the structure, morphology, and size of MnFe2O4. The as-prepared rGO/MnFe2O4 composite exhibited superior electrocatalytic activity toward the enzyme-free H2O2 detection under neutral conditions. The as-fabricated rGO/MnFe2O4/glassy carbon electrode (GCE) exhibited an excellent enzyme-free H2O2 detection with a wide linear range of 1 μM–22 mM, a lower limit of detection of 0.35 μm (S/N = 3), and a considerable sensitivity of 1180 μA mM−1 cm−2. Furthermore, rGO/MnFe2O4/GCE demonstrated the high selectivity toward H2O2 without the influence of other electroactive species and exhibited the prompt recovery in the range of 97.84–102.23% toward real sample analysis, which ensured the viable applications of rGO/MnFe2O4 composite in electrochemical enzyme-free H2O2 sensors.

ᅟ







Similar content being viewed by others
References
Y.S. Bae, S.W. Kang, M.S. Seo, I.C. Baines, E. Tekle, P.B. Chock, S.G. Rhee, J. Biol. Chem. 272, 217–221 (1997)
M. Sundaresan, Z.X. Yu, V.J. Ferrans, K. Irani, T. Finkel, Science 270, 296–299 (1995)
M.V. Marshall, L.P. Cancro, S.L. Fischman, J. Periodontol. 66, 786–796 (1995)
M.M. Sain, C. Daneault, M. Parenteau, Can. J. Chem. Eng. 75, 62–69 (1997)
H. Pelicano, D. Carney, P. Huang, Drug Resist. Updat. 7, 97–110 (2004)
Y. Chen, E.M. Ward, J. Kong, S.J. Israels, S.B. Gibson, Cell Death Differ. 5, 171–182
E.R. Whittemore, D.T. Loo, J.A. Watt, C.W. Cotman, Neuroscience 67, 921–932 (1995)
B. Thirumalraj, D. Zhao, S. Chen, S. Palanisamy, J. Colloid Interface Sci. 470, 117–122 (2016)
Y. Zhang, X. Bai, X. Wang, K. Shiu, Y. Zhu, H. Jiang, Anal. Chem. 86, 9459–9465 (2014)
J. Yang, H. Xiang, L. Shuai, S. Gunasekaran, Electrochim. Acta 708, 44–51 (2011)
M.S. Lin, H.J. Leu, Electroanalysis 17, 2068–2073 (2005)
J. Salamon, Y. Sathishkumar, K. Ramachandran, Y.S. Lee, D.J. Yoo, A.R. Kim, G.G. Kumar, Biosens. Bioelectron. 64, 269–276 (2015)
G.J. Rani, K.J. Babu, G.G. Kumar, M.A.J. Rajan, J. Alloys Compd. 688, 500–512 (2016)
J.W. Park, A.N. Jang, J.H. Song, C.Y. Park, Y.S. Lee, J. Nanosci. Nanotechnol. 13, 1895–1898 (2013)
C.L. Warner, W. Chouyyok, K.E. Mackie, D. Neiner, L.V. Saraf, T.C. Droubay, M.G. Warner, R.S. Addleman, Langmuir 28, 3931–3937 (2012)
X. Peng, J. Qu, S. Tian, Y. Ding, X. Hai, B. Jiang, M. Wuc, J. Qiub, RSC Adv. 6, 104549–104555 (2016)
J. Chen, W. Wen, L. Kong, S. Tian, F. Ding, Y. Xiong, Ind. Eng. Chem. Res. 53, 6297–6306 (2014)
C.S. Wang, N.C. Wang, Preparation and characteristics of ferrite catalysts for reduction of CO2. Mater. Chem. Phys. 88, 258–263 (2004)
B. Sahoo, S.K. Sahu, S. Nayak, D. Dhara, P. Pramanik, Catal. Sci. Technol. 2, 1367–1374 (2012)
M. Li, Y. Xiong, X. Liu, X. Bo, Y. Zhang, C. Han, L. Guo, Nano 7, 8920–8930 (2015)
L. Cao, Y. Liu, B. Zhang, L. Lu, ACS Appl. Mater. Interfaces 2, 2339–2346 (2010)
A. Yang, Y. Xue, Y. Zhang, X. Zhang, H. Zhao, X. Li, Y. He, Z. Yuan, J. Mater. Chem. B 1, 1804–1811 (2013)
T.K. Hong, D.W. Lee, H.J. Choi, H.S. Shin, B.S. Kim, ACS Nano 4, 3861–3868 (2010)
G.H. Ren, Z.S. Yu, Solid State Phenom. 181, 393–396 (2012)
J. Kim, J. Seo, J. Cheon, Y.-J. Kim, Bull. Kor. Chem. Soc. 30, 183–187 (2009)
B. Antic, A. Kremenovic, N. Jovic, M.B. Pavlovic, C. Jovalekic, A.S. Nikolic, G.F. Goya, C. Weidenthaler, J. Appl. Phys. 111(074309), 1–5 (2012)
G.G. Kumar, Z. Awan, K.S. Nahm, J.S. Xavier, Biosens. Bioelectron. 53, 528–534 (2014)
M. Vinothkannan, C. Karthikeyan, G. Gnana Kumar, A.R. Kim, D.J. Yoo, Spectrochim. Acta A Mol. Biomol. Spectrosc. 136, 256–264 (2015)
M.Y. Rafiquea, P. Li-Qing, Q. Javed, M.Z. Iqbalb, Q.H. Mei, M. Hassan Farooq, G.Z. Gang, M. Tanveerd, Chin. Phys. B 22, 107101 (2013)
P. Song, X. Zhang, M. Sun, X. Cui, Y. Lin, RSC Adv. 2, 1168–1173 (2012)
I. Kotutha, E. Swatsitang, W. Meewassan, S. Maensiri, Jpn. J. Appl. Phys. 54, 06FH10 (2015)
Z. Xing, J. Tian, A.M. Asiri, A.H. Qusti, A.O.A. Youbi, X. Sun, Biosens. Bioelectron. 52, 452–457 (2014)
F. Xu, M. Deng, G. Li, S. Chen, L. Wang, Electrochim. Acta 88, 59–65 (2013)
S. Liu, J. Tian, L. Wang, X. Sun, Carbon 49, 3158–3164 (2011)
M.Q. Wang, Y. Zhang, S.J. Bao, Y.N. Yu, C. Ye, Electrochim. Acta 190, 365–370 (2016)
M. Liu, Y.X. Yu, W.D. Zhang, Electroanalysis 28, 1–8 (2016)
F. Lorestani, Z. Shahnavaz, P. Mn, Y. Alias, N.S.A. Manan, Sensors Actuators B Chem. 208, 389–398 (2015)
M.R. Zhang, X.Q. Chen, G.B. Pan, Sensors Actuators B Chem. 240, 142–147 (2017)
A. Uzunoglu, A.D. Scherbarth, L.A. Stanciu, Sensors Actuators B Chem. 220, 968–976 (2015)
T. Marimuthu, M.R. Mahmoudian, S. Mohamad, Y. Alias, Sensors Actuators B Chem. 202, 1037–1043 (2014)
T.D. Thanh, J. Balamurugan, S.H. Lee, N.H. Kim, J.H. Lee, Biosens. Bioelectron. 85, 372–377 (2016)
Z. Yang, C.C. Qi, X. Zheng, J. Zheng, New J. Chem. 39, 9358–9362 (2015)
J. Zhang, D. Rao, J. Zheng, Electroanalysis 27, 1–9 (2015)
Y. Zhang, Z. Wang, Y. Ji, S. Liu, T. Zhang, RSC Adv. 5, 39037–39041 (2015)
S. Zhang, Q. Sheng, J. Zheng, RSC Adv. 5, 26878–26885 (2015)
Z. Yang, X. Zheng, J. Zheng, Ind. Eng. Chem. Res. (2016). https://doi.org/10.1021/acs.jecr.6b02953
Z. Li, X. Zheng, J. Zheng, New J. Chem. 40, 2115–2120 (2016)
K. Ramachandran, A. Zahoor, T.R. Kumar, K.S. Nahm, A. Balasubramani, G.G. Kumar, J. Ind. Eng. Chem. (2016). https://doi.org/10.1016/j.jiec.2016.09.012
K.J. Babu, A. Zahoor, K.S. Nahm, R. Ramachandran, M.A.J. Rajan, G.G. Kumar, J. Nanopart. Res. 16, 2250–2259 (2014)
Funding
This research was supported by the University Grants Commission Grant No.: MRP-MAJOR-CHEM-2013-36681. This work was supported by the Science and Engineering Research Board (SERB), New Delhi, India, Major Project Grant No. EMR/2015/000912.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rani, G.P.J., Saravanan, J., Sheet, S. et al. The Sensitive and Selective Enzyme-Free Electrochemical H2O2 Sensor Based on rGO/MnFe2O4 Nanocomposite. Electrocatalysis 9, 102–112 (2018). https://doi.org/10.1007/s12678-017-0418-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-017-0418-2