Abstract
Medullary thyroid cancer (MTC), an uncommon and slow-growing tumor, is difficult to eradicate when metastasis or recurrence develops. This review describes therapeutic approaches to patients with recurrent sporadic MTC, management of advanced cases of MTC, and future treatment options. A literature review of treatment of MTC in humans was conducted. Surgery is currently the only potentially curative treatment for MTC; complete tumor resection and removal of suspicious nodes is the most important initial treatment to prevent recurrence and metastasis. When recurrence or metastatic MTC develops, the decision for continued observation or initiation of systemic therapy is based on the degree of tumor aggressiveness. Lymph node involvement, calcitonin doubling time, types of RET mutation, and tumor stage are factors that help determine the need for further treatment. Therapeutic options for aggressive and inoperable MTC primarily include tyrosine kinase inhibitors, external beam radiation therapy, or other medications. Among tyrosine kinase inhibitors, vandetanib is the first drug that is FDA approved for treatment of MTC. Focused external beam radiation therapy can be reconsidered for patients with cervical node involvement. Although other targeted drug therapies have been tried, definitive clinical studies are lacking. In recurrent or advanced MTC, when systemic therapy is warranted, vandetanib is available for use in treatment; however, side effects of this drug can be problematic, and impact on overall survival is presently unknown. Newer therapeutics are being studied with the goal of balancing control of tumor growth with maintaining the patient’s quality of life.
Similar content being viewed by others
Introduction
Medullary thyroid carcinoma (MTC) is a rare malignancy arising from unregulated replication of parafollicular C cells of the thyroid gland. Accounting for only approximately 5 % of thyroid malignancies, MTC can occur in a hereditary form (multiple endocrine neoplasia 2a [MEN2a], MEN2b, familial MTC syndrome) or sporadically. In the hereditary forms and in a subset of sporadic variants, the underlying genetic defect is a missense mutation in the rearranged during transfection (RET) proto-oncogene. The purpose of this review is to discuss the adjuvant therapies either currently available or undergoing trials for the treatment of patients with recurrent or advanced disease in cases of medullary thyroid carcinoma.
Current Treatment Strategy
Surgical Procedure Determines the Outcome of MTC
Currently, surgery is the only potentially curative treatment for MTC as MTC is generally unresponsive to external beam radiotherapy (EBRT), radioiodine-131, and classic chemotherapy. Although a few studies have shown that partial thyroidectomy may be sufficient for a specific population of patients, it is generally accepted that total thyroidectomy with lymph node dissection is required. Based on current American Thyroid Association (ATA) guidelines, the extent of surgery should be determined by the presence or absence of local or distant metastasis [1]. With appropriate surgery, a biochemical cure rate of 42.9 % may be achieved [2]. The extent of lymph node dissection remains controversial. During surgical neck exploration, lymph node metastases are common and reported in 55 % of patients [3]. The presence of cervical lymph node involvement predicts ipsilateral and contralateral nodal disease, local recurrence, and distant metastasis [3–6]. Therefore, preoperative neck imaging, including the central compartment nodes, is crucial. The presence of nodal disease or a serum calcitonin level greater than 400 pg/ml requires neck computed topography (CT), chest CT, liver protocol CT, or magnetic resonance imaging (MRI) as recommended by the ATA guidelines [1]. Remnant tumor tissue following surgical resection can be a source for recurrent or metastatic disease as it may contain premature embryonic components [7, 8], as well as the RET proto-oncogene mutation [9]. Despite data clearly demonstrating an association of long-term outcomes with adequacy of tumor resection, variable practice patterns exist in the USA. Patient characteristics (age <65, female gender), geographic location, and tumor size predict for discordance between the extent of surgery and lymph node dissection the patient received and that recommended by ATA guidelines [10]. Those receiving surgery that differs from ATA guidelines had statistically lower survival rates [10]. It is therefore recommended that patients with MTC, whether familial or sporadic, have a total thyroidectomy with appropriately aggressive lymph node dissection as first-line therapy.
Selection of Patients for Drug Therapy
Since MTC is generally slow growing, most patients can be observed without aggressive treatment even in the presence of metastasis. How can we determine which patients are candidates for additional systemic treatment or clinical trials? Table 1 lists prognostic factors in aggressive MTC. The presence of these characteristics can help to identify patients that may benefit from more aggressive therapy. In the absence of locoregionally confined MTC, prediction of survival outcome using tumor and clinical characteristics becomes important in determining the role of systemic therapies after surgical resection. The most important predictors of outcome are stage of disease and age at time of diagnosis. If metastases are present at the time of diagnosis, the 10-year survival rate is 40 % contrasted with 95.6 % in those with disease confined to the thyroid gland [11]. For each additional year of age, there is a 5.2 % increase in risk of death with prognosis in those over age 65 being especially poor [11]. Another means of predicting survival includes the use of serum calcitonin doubling time. When the doubling time was longer than 2 years, no deaths were reported; however, when doubling time was less than 6 months, the 5-year survival rate was 25 % [12]. Caution must be exercised when using doubling time, as fluctuations in serum calcitonin levels may mimic worsening or improving MTC [13]. Radiographic features can also predict progressive disease. An increase in the sum of the long diameter of target lesions of 20 % or more in less than 1 year is considered as progressive (Response Evaluation Criteria In Solid Tumors (RECIST) criteria), and in this setting, additional therapy may be considered. Although missense in the RET proto-oncogene underlies the majority of MTC cases, all RET mutations do not confer the same risk of poor outcomes. The ATA guidelines categorize the highest risk in germline mutation of RET to be A918T, A883F, and the rare combination of V804M with second sites in the RET gene (Table 1). These mutations are seen only in patients with MEN2b. The second highest risk involves mutations at codon 634 in exon 11: C634R, C634G/F/S/W/Y [1]. Surprisingly, somatic mutations were present in 43 % of patients with sporadic MTC; mutation A918T occurs in 79 % of those with a mutation [14]. The presence of somatic mutations correlated with poorer clinical course and survival [14]. Once survival outcome is determined, additional therapies including additional surgery, drug therapy, or EBRT can be considered.
Table 2 summarizes treatment methods applied to patients with MTC. The efficacy of each treatment and adverse events grade 3 or higher are listed, since serious drug side effects have to be clearly recognized. Drugs having a low response rate (e.g., doxorubicin) are not listed.
Novel Therapies
Patients with aggressive MTC are candidates for further treatment with medical therapy including tyrosine kinase inhibitors, radiotherapy, and chemotherapy. The currently available drugs for treatment or use in clinical trials are the tyrosine kinase inhibitors (TKI).
Tyrosine Kinase Inhibitors
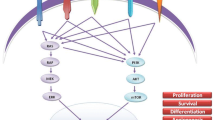
The constitutively activated RET proto-oncogene and vascular endothelial growth factor receptor (VEGFR) are the primary targets of tyrosine kinase inhibitors in the treatment of MTC. Overexpression of VEGFR 1 and 2 has been shown to be present in MTC tissue [15]. Interestingly, tyrosine kinase inhibitors demonstrate individual specificity towards the type of RET mutation in vitro; this suggests that mutation-specific therapies may be applicable in the treatment of MTC [16]. Various kinase inhibitors, their efficacy, and grade 3 or higher side effects are shown in Table 2.
Vandetanib
In April 2011, vandetanib (Caprelsa from AstraZeneca Pharmaceuticals LP, Wilmington, DE) became the first US FDA-approved tyrosine kinase inhibitor for treatment of progressive or symptomatic MTC. This drug selectively inhibits RET proto-oncogene, VEGFR-2, and EGFR-dependent signaling. In its phase II study, 30 patients with germline mutations (hereditary MTC) received oral vandetanib starting at 300 mg/day with dose adjustments based on clinical response and drug toxicity [17]. At study entry, 29 patients had distant metastases to the liver, lymph nodes, and lung. Objective tumor response was determined at 12-week intervals through serial imaging with either MRI or CT. Twenty percent of participants achieved partial response at study cutoff, with a median duration of 10.2 months, and 53 % achieved a durable disease state (i.e., stable disease for ≥24 weeks). The overall disease control rate was 73 %, and expected progression-free survival was 27 months. Almost all patients showed reduction in serum calcitonin and CEA levels. Drug-related side effects were common with 56 % incidence of grade 3 side effects [17]. In a second phase II study, Robinson et al. evaluated the efficacy of using a lower dose of vandetanib (100 mg) in 19 patients with hereditary MTC [18]. A 16 % objective tumor response rate with median duration of 6 months was observed. Median progression-free survival (PFS) could not be determined. No patients developed grade 3 or higher adverse events; however, two patients discontinued the study due to drug-related side effects, and two patients required a reduced dose. In addition, only 16 % of patients showed at least 50 % decrease in calcitonin from baseline [18]. In the phase III trial (ZETA), 331 patients with germline or unknown mutation status were randomized to receive either vandetanib 300 mg daily (n = 231) or placebo (n = 100) for 24 months [19]. In addition, at data cutoff, 93 eligible patients, from both treatment and placebo arms, with progressive disease entered open-label treatment with vandetanib. A 54 % reduction in risk of progression with estimated median PFS of 30 months was seen in the vandetanib arm. In addition, results for progression-free disease at 6 months showed 83 % survival in the treatment group compared to 63 % in the placebo groups. Analysis of intention to treat initially included data from the 93 patients who participated in the open-label study; however, after exclusion of these patients, the PFS hazard ratio was 0.27 (p < 0.0001). Objective tumor response rate was 45 % compared to 13 % in the placebo arm, but after exclusion of the open-label data, tumor response rate was 44 and 1 %, respectively. Overall, survival rate at time of data cutoff was not statistically different between either arm with HR of 0.89 and 95 % CI of 0.48–1.65. Whereas 69 % of those on vandetanib achieved a minimum of 50 % decrease in calcitonin and 52 % achieved reduction in CEA, only 3 and 2 %, respectively, in the placebo arm achieved these measures. Nearly all patients randomized to vandetanib reported at least one side effect with more than half reporting grade 3 or higher events. The most common symptoms were diarrhea, rash, hypertension, nausea, and fatigue. Of the grade 3 or higher events, QT prolongation was reported in 8 % of patients receiving vandetanib compared to 1 % on placebo. Because of the significant cardiac toxicities of vandetanib, baseline electrocardiogram should be obtained to evaluate for conduction anomalies that may predispose to fatal cardiac arrhythmia [19].
Axitinib
Axitinib is an oral selective inhibitor of VEGFR 1, 2, and 3 as well as a less potent inhibitor of platelet-derived growth factor (PDGF) and c-KIT. Unlike other TKIs, axitinib does not have activity against RET. In a phase II trial, 60 patients with thyroid cancer received axitinib initiated at 5 mg twice daily with either uptitration of dose if well tolerated or reduced dose in the setting of greater than or equal to grade 2 toxicity [20]. Only 2 (18 %) of 11 patients with metastatic MTC showed a partial response, and only three (27 %) patients achieved stable disease. PFS was not determined for each histiologic subtype, but overall median PFS was 18.1 months. The most common side effects reported included diarrhea, nausea, anorexia, fatigue, hypertension, stomatitis, weight loss, and headache. Grade 3 or higher adverse events were relatively common with 32 % reporting at least one event. Three patients developed a grade 4 adverse event (stroke, reversible posterior leukoencephalopathy syndrome, or proteinuria); however, in all cases, toxicity was resolved [20].
Motesanib
Motesanib is a potent inhibitor of c-KIT, PDGF, and VEGFR 1, 2, and 3 with activity against wild-type RET in vitro as demonstrated by studies in mice. In a phase I trial, one patient with MTC was enrolled and achieved a partial response [21]. In its phase II trial, 91 patients with MTC received a self-administered oral daily dose of 125 mg motesanib for up to 48 months [22]. Eighty-four percent of patients had sporadic MTC, and 93 % had metastatic disease. A low rate of partial response (2 %) was observed; however, a high rate of stable disease (81 %) and durable stable disease ≥24 weeks (48 %) was observed. Median PFS was 48 weeks. Tumor size reduction was seen in 76 %. Reduction in serum calcitonin and CEA occurred in 83 and 75 %, respectively. The majority of patients (88 %) developed at least one adverse effect with 41 % developing a grade 3 or 4 adverse event. Common side effects reported include fatigue, hypertension, nausea, anorexia, and diarrhea [22].
Cabozantinib
Cabozantinib is an oral multikinase inhibitor of VEGFR 2, RET, and MET. In a phase I trial, 85 patients were enrolled to determine the safety of the drug and its maximum tolerable dose [23]. Thirty seven of 85 patients had a diagnosis of advanced MTC with 71 % having sporadic disease. Confirmed partial response was seen in 29 % of patients, and stable disease of at least 6 months’ duration was seen in 41 %. Median PFS could not be determined at time of data analysis. Reduction in calcitonin was variable, ranging from 3 to 99 %; for those in which data for CEA were available, a reduction from 13 to 94 % was seen. The majority of patients reported at least one drug-related adverse effect with 43 % reporting grade 1 or 2 events. The most frequently reported events included diarrhea, nausea, anorexia, fatigue, rash, palmar–plantar erythrodysesthesia, increased AST, and mucositis. One grade 4 event (pulmonary embolism) was reported [23]. A phase III study, drug vs. placebo, is currently ongoing.
Sorafenib
Sorafenib is currently FDA approved for use in the treatment of renal cell and hepatocellular carcinomas. It has a wide range of inhibitory activity against Raf, platelet-derived growth factor receptor B, FLT-3, c-kit, VEGFR 2 and 3, as well as RET. In both in vivo and in vitro models, sorafenib has demonstrated inhibition of both wild-type and mutant RET kinases and growth of MTC cells. In a phase II trial, 21 patients with MTC, of which 16 had sporadic disease, with evidence of metastasis or advanced local disease received sorafenib 400 mg twice a day [24]. The median duration of therapy was 15 months. Unfortunately, due to slow accrual, the hereditary MTC study arm was prematurely terminated. Of the 16 sporadic MTC cases, 1 patient showed partial response (6.3 %) and 14 patients achieved stable disease (87.5 %) of which 8 patients achieved durable stable disease for ≥15 months. Although overall survival was not reached at time of data analysis, the mean PFS was 17.9 months. In all patients with A918T somatic mutation, sorafenib maintained a stable disease state. Among the group, tumor shrinkage was variable from 8 to 27 %. However, the majority of patients experienced a decrease in calcitonin, CEA, or both. Although sorafenib was reasonably well tolerated, with low-grade adverse effect rates similar to other TKIs, rare but serious toxicities (e.g., bowel perforation) and one death were reported [24]. In another phase II trial, 34 patients, of which 15 had locally advanced or metastatic MTC, were started on sorafenib 400 mg twice daily until disease progression with median duration of follow-up of 19 months [25]. Within the MTC subgroup, three had germline RET mutations and six had RET mutation without genomic alteration; in six, RET status was unknown. Overall, radiologic response rate for the MTC subgroup at 6 and 12 months was 13 and 25 %, respectively. Partial response and stable disease rates were not reported based on histology. Median PFS and overall survival were not determined at time of data analysis; however, at 1 year, PFS was 93 % and overall survival was 100 %. The mean calcitonin level decreased by greater than 50 % in the first month, which increased and reached a plateau in months 2 to 3. CEA levels also initially decreased but increased after 9 months. Seventy-nine percent of patients required a 50 % dose reduction while a third of patients required dose reduction to 400 mg every other day. The majority of patients developed dermatologic complications including 79 % developing hand–foot syndrome (HFS). Other grade 3 or 4 adverse events included diarrhea, fatigue, infection, mucositis, arthralgia, constipation, hypertension, depression, headache, anemia, fever, and myalgias. An idiosyncratic effect of sorafenib was a rise in TSH seen in 12 % of patients. Although the exact mechanism remains unclear, inference from prior studies suggests that sorafenib interferes with metabolism of T4 and T3 [25]. In a phase I trial of sorafenib in combination with tipifarnib, 35 patients including 13 with MTC were given sorafenib 400 mg in the morning and 200 mg in the evening with tipifarnib either 200 or 100 mg twice daily [26]. Sorafenib and tipifarnib were dosed on a schedule of 21 days on and 7 days off. Three of the 13 MTC patients had determined germline RET mutations. The partial response rate was 38 %, and stable disease of at least 6 months’ duration was achieved in 31 %. Median PFS was 15 months, and overall survival at 24 months was 88 %. All patients with a partial response demonstrated a decrease in calcitonin. Grade 3 or higher adverse events included rash, fatigue, diarrhea, and elevation in lipase and amylase and were responsible for dose reduction in 6 of 14 patients [26].
Sunitinib
Sunitinib is an oral kinase inhibitor of RET, VEGFR 2, PDGF, FLT3, and c-KIT. It is currently FDA approved in the treatment of renal cell carcinoma and gastrointestinal stroma tumors. In a phase II trial of 23 MTC patients with progressive disease, 11 patients had RET mutations (eight somatic and three germline mutations). Fifty milligrams of sunitinib was given on an intermittent dosing schedule with 4 weeks on and 2 weeks off [27]. A partial response was achieved in 35 % and stable disease in 57 % of patients. Seven of nine patients having A918T mutation showed either partial response or stable disease. Although median PFS was not determined, the median time to progression was 12.8 months. The most common treatment-related adverse events were fatigue, lymphopenia, neutropenia, nausea, diarrhea, mucositis, and palmar–plantar erythema (PPE) [27]. In another phase II trial of seven patients with FDA-PET-positive metastatic MTC, sunitinib 37.5 mg daily was given continuously unless disease progression, unacceptable toxicity, or patient withdrawal occurred [28]. Fifty percent of patients achieved RECIST response. Grade 3 or 4 event rates were significant; gastrointestinal bleeding and hemoptysis with one treatment-related death were the most serious and may be related to concomitant use of anticoagulation [28].
Summary and Practical Use of TKI for Patients with MTC
The development of tyrosine kinase inhibitors has brought new hope for the management of aggressive MTC. In MTC, the effect of TKIs appears to be primarily mediated by inhibition of VEGF signaling and, to a slightly lesser extent, inhibition of RET. In most cases, use of TKIs resulted in attaining a stable disease state more often than regression of disease; however, in the phase III trial of vandetanib, an objective tumor response was seen in 45 % of patients [19], making this the current TKI of choice in advanced MTC. TKIs have shown promise in prolonging the PFS, although overall survival rates at 5 or 10 years have yet to be determined. Use of TKIs is currently limited by their significant adverse drug profiles with most patients experiencing grade 1–2 adverse events and, in the rare cases, grade 3 or higher event. As the pathogenesis of MTC is better understood, TKIs and other targeted gene therapies may come to play a more prominent role in the treatment of aggressive MTC. In the meantime, surgery remains the cornerstone of therapy.
Capecitabine
In general, chemotherapeutic agents are not overwhelmingly effective in the treatment of MTC [29]. There are, however, sporadic case reports of successful control of metastatic MTC with use of capecitabine [30–32]. Capecitabine is currently FDA approved for use in patients with metastatic breast cancer. Capecitabine is a carbamate derivative of doxifuridine that is converted into 5-fluorouracil in the presence of thymidine phosphorylase at the tumor site, resulting in inhibition of DNA synthesis. Since it is active on rapidly proliferating tumor cells, it is feasible that capecitabine may have a therapeutic role in aggressive MTC. Common grade 3 side effects of capecitabine are hand–foot syndrome and diarrhea.
Indomethacin
Indomethacin, a nonselective cyclooxygenase inhibitor of prostaglandin synthesis, has demonstrated antitumor effects in various cancer cell lines [33–37]. In particular, the anti-proliferative effect of indomethacin has been shown on MTC cells in nude mice models [38, 39]. In a MTC cell line, indomethacin reduces S phase progression by inhibiting phosphorylated Rb protein expression [40]. Although in vitro studies of indomethacin have been promising, clinical experience with use of indomethacin in patients with recurrent or metastatic MTC has been limited to three cases. In two patients with widely metastatic disease, indomethacin therapy for 3 or 4 months caused marked reduction in tumor mass as well as calcitonin level; however, in a third patient with MEN IIa and metastatic disease to the lungs, therapy with indomethacin did not result in a clinical response [13]. The efficacy of indomethacin in MTC patients has yet to be determined by clinical trials.
External Beam Radiation Therapy
Poor survival rates initially reported with EBRT made this therapeutic modality unfavorable in the treatment of MTC [41]. Further evaluation of previous reports, however, revealed a lack of information regarding the particular surgical procedure employed prior to radiation therapy. As stated previously, the extent of surgical resection determines the clinical course in the treatment of MTC [10]. Martinez et al. revisited the efficacy of EBRT on selected patients with MTC from the Surveillance, Epidemiology and End Results database; they chose 66 patients who received EBRT following total thyroidectomy and lymphadenectomy [42]. In the univariate analysis, there was no significant improvement in overall survival after 12 years in those patients receiving EBRT when compared with those without EBRT; however, a survival benefit was seen in patients with positive neck nodes. In the multivariate analysis of node-positive patients, EBRT did not influence overall survival [42]. In retrospective studies, EBRT did not show survival benefit in high-risk patients probably because of the high rate of microscopic metastasis in high-risk patients. EBRT did, however, demonstrate improvement in locoregional disease control in patients at high risk for locoregional relapse with a 5-year relapse-free rate of 87 % and 5-year survival rate of 56 % [43]. In addition to the lack of survival benefit of EBRT in MTC, concern for toxicity has limited the use of adjuvant conventional EBRT. With advances in EBRT namely intensity-modulated radiation therapy (IMRT), it is possible to deliver high doses of radiation to the thyroid bed with sparing of normal surrounding tissues. Most patients undergoing IMRT have reported acute radiotherapy toxicity; however, these side effects are manageable [44]. Based on current data, EBRT should also be considered in patients at high risk for metastatic neck node recurrence when extensive surgical resection is not possible. As per ATA guidelines, patients with incomplete tumor resection despite optimal surgery should be considered for adjuvant EBRT to the neck and mediastinum (grade B recommendation) [1]. In addition, patients with moderate- to high-volume central compartment involvement with either unilateral or bilateral level 2A-V nodal disease should also be considered for EBRT following optimal surgery if microscopic positive margins are present (grade C recommendation) [1].
Radioimmunotherapy
In a study by the French Endocrine Tumor Group, 29 patients with advanced and progressive MTC received a biospecific anti-CEA monoclonal antibody followed by an 131I-labeled hapten compared to 39 high-risk untreated patients [45]. Overall survival did not differ between the treatment and control groups; however, in the subgroup analysis of patients with calcitonin doubling times <2 years, significant improvement in overall survival was observed (110 vs. 61 months; p < 0.03). Toxicity was mostly hematologic and associated with bone marrow involvement [45].
Receptor Radionuclide Therapy
MTC cells originate from the neural crest that secretes a number of proteins including somatostatin, CEA, calcitonin, neuron-specific enolase, chromogranin, and gastrin-releasing peptide. Yttrium 90-labeled somatostatin analog [90Y-DOTATOC] has been tried for patients with advanced MTC. The rationale for this treatment is that MTC cells not only produce somatostatin but also express corresponding receptors on their membrane [46, 47]. Thus, uptake of radio-labeled somatostatin analog through somatostatin receptors should cause beta irradiation in MTC cells. Bodei et al. gave 7.5 to 19.2 GBq of 90Y-DOTATOC in two to eight cycles to 21 patients with advanced MTC [48]. Complete response was seen in 2 of the 21 patients (10 %), and stabilization of disease was seen in 57 % patients based on tumor image analysis. The response rate was dependent on degree of octreotide receptor expression, tumor size, and calcitonin level. With the exception of one patient who developed a grade 3 hematological side effect, 90Y-DOTATOC was well tolerated [48].
Perspectives for the Future
The treatment for recurrent or advanced MTC remains an area for further research. There is a need for effective drugs with minimal side effects and for a better understanding of the role of radiotherapy. In developing new therapeutics, the patient’s quality of life is an important factor to consider. One area to be explored is that of immunotherapy or cytokine therapy. Local injection of free IL-2 into cancer tissues has been described in humans with some success. Direct injection of free IL-2 into tumor tissue leads to hemorrhagic necrosis and subsequent activation of tumor immunity by lymphocyte stimulation [49]. This may, in the future, be appropriate for use in patients with MTC who require rapid reduction in tumor burden. Another cytokine-directed therapy includes leukemia inhibitory factor (LIF). LIF is a multifunctional cytokine of the interleukin-6 family dependent on Ras/Raf activation for induction and secretion [50]. In papillary thyroid carcinoma, activation of Ras/Raf leads to cancer cell proliferation. In contrast, activation of Ras/Raf inhibits MTC cell growth by secretion of LIF which downregulates the RET proto-oncogene [51]. Recombinant LIF appears to be safe for use in humans [52] and may in the future prove effective in treatment of MTC.
Two additional prospective agents include atrial natriuretic hormone (ANP) derivatives and tetraiodothyroacetic acid. In cell culture, ANP derivatives have demonstrated rapid reduction in MTC cell volume [53]. The antitumor effects of ANP derivatives appear to be inhibition of angiogenesis and induction of tumor suppressor gene Rb [54]. Although ANPs have been used in humans [55], this agent has not been tried in patients with MTC. Tetraiodothyroacetic acid is a deaminated analog of l-thyroxine. In a preclinical trial, tetraiodothyroacetic acid inhibited MTC cell growth by inducing apoptosis [56].
Conclusions
Initial management of MTC continues to be surgical resection with appropriate nodal dissection to avoid recurrence and metastasis. Once inoperable metastasis occurs, determination of whether patients can continue with observation or require systemic treatment is based on the calcitonin doubling time and tumor progression using the RECIST criteria. If patients require systemic treatment, vandetanib is now FDA approved for use in the treatment of advanced MTC. Although other TKIs, chemotherapy, radiotherapy, and radioimmune and radionuclide therapies have shown effects against MTC cells either in vitro or in vivo, more effective therapies with fewer side effects remain to be established.
References
Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Fharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA Jr (2009) Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19:565–612
Pelizzo MR, Boschin IM, Bernaante P, Toniato A, Piotto A, Pagetta C, Nibale O, Rampin L, Muzzio PC, Rubello D (2007) Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol 33:493–497
Scollo C, Baudin E, Travagli JP, Caillou B, Bellon N, Leboulleux S, Schlumberger M (2003) Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab 88:2070–2075
Machens A, Hauptmann S, Dralle H (2008) Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg 95:586–591
Weber T, Schilling T, Frank-Raue K, Colombo-Benkmann M, Hinz U, Ziegler R, Klar E (2001) Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery 130:1044–1049
Ito Y, Miyauchi A, Yabuta T, Fukushima M, Inoue H, Tomoda C, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F (2009) Alternative surgical strategies and favorable outcomes in patients with medullary thyroid carcinoma in Japan: experience of a single institution. World J Surg 33:58–66
Westerlund J, Andersson L, Carlsson T, Zoppoli P, Fagman H, Nilsson M (2008) Expression of Islet1 in thyroid development related to budding, migration, and fusion of primordia. Dev Dyn 237:3820–3829
Zhang P, Zuo H, Ozaki T, Nakagomi N, Kakudo K (2006) Cancer stem cell hypothesis in thyroid cancer. Pathol Int 56:485–489
Lanzi C, Cassinelli G, Nicolini V, Zunino F (2009) Targeting RET for thyroid cancer therapy. Biochem Pharmacol 77:297–309
Panigrahi B, Roman SA, Sosa JA (2010) Medullary thyroid cancer: are practice patterns in the United States discordant from American Thyroid Association Guidelines? Ann Surg Oncol 17:1490–1498
Roman S, Lin R, Sosa JA (2006) Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107:2134–2142
Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF, GTE Study Group (2005) Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 90:6077–6084
Sugawara M, Geffner DL, Martinez D, Hershman JM (2009) Novel treatment of medullary thyroid cancer. Curr Opin Endocrinol Diabetes Obes 16:367–372
Elisei R, Cosci B, Romei C, Bottici V, Renzini F, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, Miccoli P, Berti P, Pacini F, Pinchera A (2008) Prognostic significance of somatic ret oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 93:682–687
Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL (2010) Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid 20:863–871
Verbeek HH, Alves MM, de Groot JW, Osinga J, Plukker JT, Links TP, Hofstra RM (2011) The effects of four different tyrosine kinase inhibitors on medullary and papillary thyroid cancer cells. J Clin Endocrinol Metab 96:E991–E995
Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M (2010) Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28:767–772
Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R (2010) Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab 95:2664–2671
Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ (2012) Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer (MTC): a randomized, double-blind phase III trial. J Clin Oncol 30:134–141
Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinksy DR, Liau KF, Cohen RB (2008) Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26:4708–4713
Rosen LS, Kurzrock R, Van Mulay M, Vugt A, Purdom M, Ng C, Silverman J, Koutsoukos A, Sun Y, Bass MB, Xu Y, Polverino A, Wiezorek JS, Chang DD, Benjamin R, Herbst RS (2007) Safety, pharmokinetics and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 25:2369–2376
Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JP, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Sherman SI (2009) Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol 27:3794–3801
Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Muller T, Ratain MN, Salgia R (2011) Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29:2660–2666
Lam ET, Ringel MD, Kloos RT et al (2010) Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 28:2323–2330
Ahmed M, Barbachano Y, Riddell A, Hickey J, Newbold KL, Viros A, Harrington KJ, Marais R, Nutting CM (2011) Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in the UK based population. Eur J Endocrinol 165:315–322
Hong DS, Cabanillas ME, Wheler J, Naing A, Tsimberidou AM, Ye L, Waguespack SG, Hernandez M, El Naggar AK, Bidyasar S, Wright J, Sherman AI, Kurzrock R (2011) Inhibition of the Ras/Raf/MEK/ERk and RET kinase pathways with the combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in medullary and differentiated thyroid malignancies. J Clin Endocrinol Metab 96:997–2005
De Souza JA, Busaidy N, Zimrin A, Seiwert TY, Villaflor VM, Poluru KB, Reddy PL, Nam J, Vokes E, Cohen EE (2010) Phase II trial of sunitinib in medullary thyroid cancer (MTC). J Clin Oncol 28:15s (suppl; abstr 5504)
Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG (2010) Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 16:5260–5268
Pacini F, Castagna MG, Cipri C, Schlumberger M (2010) Medullary thyroid carcinoma. Clin Oncol 22:475–485
Labidi SI, Gravis G, Tarpin C, Brun V, Viens P (2007) Medullary thyroid cancer treated by capecitabine. Anticancer Drugs 18:831–834
Paiva CE, Michelin OC (2008) Use of capecitabine in refractory metastatic medullary thyroid carcinoma. Thyroid 18:587
Gilliam LK, Kohn AD, Lalani T, Swanson PE, Vasko V, Patel A, Livingston RB, Pickett CA (2006) Capecitabine therapy for refractory metastatic thyroid carcinoma: a case series. Thyroid 16:801–810
Wang HM, Zhang GY (2005) Indomethacin suppresses growth of colon cancer via inhibition of angiogenesis in vivo. World J Gastroenterol 11:340–343
Huang RH, Chai J, Tarnawski AS (2006) Identification of specific genes and pathways involved in NSAIDs-induced apoptosis of human colon cancer cells. World J Gastroenterol 12:6446–6452
Kundu N, Walser TC, Ma X, Fulton AM (2005) Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol Immunother 54:981–987
He H, Xia HH, Wang JD, Gu Q, Lin MC, Zou B, Lam SK, Chan AO, Yuen MF, Kung HF, Wong BC (2006) Inhibition of human telomerase reverse transcriptase by nonsteroidal antiinflammatory drugs in colon carcinoma. Cancer 106:1243–1249
de Groot DJ, van der Deen M, Le TK, Regeling A, de Jong S, de Vries EG (2007) Indomethacin induces apoptosis via a MRP1-dependent mechanism in doxorubicin-resistant small-cell lung cancer cells overexpressing MRP1. Br J Cancer 97:1077–1083
Quidville V, Segond N, Pidoux E, Cohen R, Jullienne A, Lausson S (2004) Tumor growth inhibition by indomethacin in a mouse model of human medullary thyroid cancer: implication of cyclooxygenases and 15-hydroxyprostaglandin dehydrogenase. Endocrinology 145:2561–2571
Quidville V, Segond N, Lausson S, Frenkian M, Cohen R, Jullienne A (2006) 15-Hydroxyprostaglandin-dehydrogenase is involved in anti-proliferative effect of non-steroidal anti-inflammatory drugs COX-1 inhibitors on a human medullary thyroid carcinoma cell line. Prostaglandins Other Lipid Mediat 81:14–30
Tomoda C, Moatamed F, Naeim F, Hershman JM, Sugawara M (2008) Indomethacin inhibits cell growth of medullary thyroid carcinoma by reducing cell cycle progression into S phase. Exp Biol Med (Maywood) 233:1433–1440
Samaan NA, Schultz PN, Hickey RC (1988) Medullary thyroid carcinoma: prognosis of familial versus sporadic disease and the role of radiotherapy. J Clin Endocrinol Metab 67:801–805
Martinez SR, Beal SH, Chen A, Chen SL, Schneider PD (2010) Adjuvant external beam radiation for medullary thyroid carcinoma. J Surg Oncol 102:175–178
Schwartz DL, Rana V, Shaw S, Yazbeck C, Ang KK, Morrison WH, Rosenthal DI, Hoff A, Evans DB, Clayman GL, Garden AS, Sherman SI (2008) Postoperative radiotherapy for advanced medullay thyroid cancer-local disease control in the modern era. Head Neck 30:883–888
Rosenbluth BD, Serrano V, Happersett L, Shaha AR, Tuttle RM, Narayana A, Wolden SL, Rosenzweig KE, Chong LM, Lee NY (2005) Intensity-modulated radiation therapy for the treatment of nonanaplastic thyroid cancer. Int J Radiat Oncol Biol Phys 63:1419–1426
Chatal JF, Campion L, Kraeber-Bodéré F, Bardet S, Vuillez JP, Charbonnel B, Rohmer V, Chang CH, Sharkey RM, Goldenberg DM, Barbet J (2006) French Endocrine Tumor Group. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol 24:1705–1711
Kwekkeboom DJ, Reubi JC, Lamberts SW, Bruining HA, Mulder AH, Oei HY, Krenning EP (1993) In vivo somatostatin receptor imaging in medullary thyroid carcinoma. J Clin Endocrinol Metab 76:1413–1417
Mato E, Matías-Guiu X, Chico A, Webb SM, Cabezas R, Berna L, De Leiva A (1998) Somatostatin and somatostatin receptor subtype gene expression in medullary thyroid carcinoma. J Clin Endocrinol Metab 83:2417–2420
Bodei L, Handkiewicz-Junak D, Grana C, Mazzetta C, Rocca P, Bartolomei M, Lopera Sierra M, Cremonesi M, Chinol M, Macke HR, Paganelli G (2004) Receptor radionuclide therapy with 90Y-DOTATOC in patients with medullary thyroid carcinomas. Cancer Biother Radiopham 19:65–71
Den Otter W, Jacobs JJ, Battermann JJ, Hordijk GJ, Krastev Z, Moiseeva EV, Stewart RJ, Ziekman PG, Koten JW (2008) Local therapy of cancer with free IL-2. Cancer Immunol Immunother 57:931–950
Auernhammer CJ, Melmed S (2000) Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev 21:313–345
Arthan D, Hong SK, Park JI (2010) Leukemia inhibitory factor can mediate Ras/Raf/MEK/ERK-induced growth inhibitory signaling in medullary thyroid cancer cells. Cancer Lett 297:31–41
Davis ID, Kiers L, MacGregor L, Quinn M, Arezzo J, Green M, Rosenthal M, Chia M, Michael M, Bartley P, Harrison L, Daly M (2005) A randomized, double-blinded, placebo-controlled phase ii trial of recombinant human leukemia inhibitory factor (rhuLIF, Emfilermin, AM424) to prevent chemotherapy-induced peripheral neuropathy. Clin Cancer Res 11:1890–1898
Eichelbaum EJ, Vesely BA, Alli AA, Sun Y, Gower WR Jr, Vesely DL (2006) Four cardiac hormones eliminate up to 82 % of human medullary thyroid carcinoma cells within 24 hours. Endocrine 30:325–332
Kong X, Wang X, Xu W, Behera S, Hellermann G, Kumar A, Lockey RF, Mohapatra S, Mohapatra SS (2008) Natriuretic peptide receptor A as a novel anticancer target. Cancer Res 68:249–256
Bierwolf C, Burgemeister A, Lüthke K, Born J, Fehm HL (1998) Influence of exogenous atrial natriuretic peptide on the pituitary-adrenal response to corticotropin-releasing hormone and vasopressin in healthy men. J Clin Endocrinol Metab 83:1151–1157
Yalcin M, Dyskin E, Lansing L, Bharali DJ, Mousa SS, Bridoux A, Hercbergs AH, Lin HY, Davis FB, Glinsky GV, Glinskii A, Ma J, Davis PJ, Mousa SA (2010) Tetraiodothyroacetic acid (Tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J Clin Endocrinol Metab 95:1972–1980
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugawara, M., Ly, T. & Hershman, J.M. Medullary Thyroid Cancer—Current Treatment Strategy, Novel Therapies and Perspectives for the Future. HORM CANC 3, 218–226 (2012). https://doi.org/10.1007/s12672-012-0119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-012-0119-5