Abstract
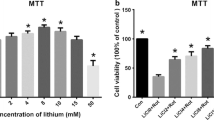
The proteasome inhibition and mitochondrial dysfunction are involved in pathomechanism of Parkinson’s disease. The main aim of this study was to assess how particular culture conditions of human dopaminergic neuroblastoma SH-SY5Y cells could affect the extent of neurodegeneration induced by proteasome inhibitor—lactacystin (LC) and mitochondrial toxin—rotenone (Rot). This study revealed that induction of neuronal differentiation of SH-SY5Y cells with retinoic acid (RA-SH-SY5Y) caused a higher resistance of these cells to LC-evoked cell death when compared to undifferentiated cells (UN-SH-SY5Y). In contrast, RA-SH-SY5Y cells were more vulnerable than the UN-SH-SY5Y to Rot-induced cell damage. Furthermore, we found that a prolonged incubation of the cells under low serum condition (PLSC) significantly increased the LC toxicity in both differentiated and undifferentiated cells. Next, the effects of combined treatment with LC and Rot on cell viability were studied in RA-SH-SY5Y cells under PLSC and normal low serum condition (NLSC). At a low concentration, Rot (0.001–1 μM) attenuated the LC-evoked cell death in RA-SH-SY5Y cells exposed to NLSC. In contrast, under PLSC low concentrations of Rot lacked neuroprotective action while its higher levels (10 μM) enhanced the LC toxicity. Further, we showed that low concentrations of celastrol (Cel; 0.001 μM), a putative neuroprotective agent with antioxidant and anti-inflammatory properties, were able to partially attenuate the Rot-evoked toxicity under both PLSC and NLSC. On the other hand, Cel (0.001 and 0.01 μM) attenuated the LC-induced cell damage only under PLSC. Interestingly, higher concentrations of Cel (>1 μM) reduced cell viability in both UN- and RA-SH-SY5Y but only in UN-SH-SY5Y cells the effect was enhanced under PLSC. The obtained data indicate that toxicity of LC and Rot in SH-SY5Y cell line depends on the stage of cell differentiation and is enhanced in cells cultured for a longer time in low serum medium. Moreover, the neuroprotective properties of Rot and Cel against the LC-induced cell damage can be observed only under particular low serum conditions.








Similar content being viewed by others
Abbreviations
- Cel:
-
Celastrol
- LC:
-
Lactacystin
- LDH:
-
Lactate dehydrogenase
- MTT:
-
3-[4,5-Dimethylthylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- NLSC:
-
Normal low serum condition
- PLSC:
-
Prolonged low serum condition
- RA-SH-SY5Y:
-
Retinoic acid-differentiated SH-SY5Y cells
- Rot:
-
Rotenone
- UN-SH-SY5Y:
-
Undifferentiated SH-SY5Y cells
References
Agholme L, Lindström T, Kågedal K, Marcusson J, Hallbeck M (2010) An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis 20:1069–1082
Andoh T, Chock PB, Chiueh CC (2002) The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J Biol Chem 277:9655–9660
Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT (2006) Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis 22:404–420
Cardoso SM, Moreira PI, Agostinho P, Pereira C, Oliveira CR (2005) Neurodegenerative pathways in Parkinson’s disease: therapeutic strategies. Curr Drug Targets CNS Neurol Disord 4:405–419
Cartier AE, Ubhi K, Spencer B, Vazquez-Roque RA, Kosberg KA, Fourgeaud L, Kanayson P, Patrick C, Rockenstein E, Patrick GN, Masliah E (2012) Differential effects of UCHL1 modulation on alpha-synuclein in PD-like models of alpha-synucleinopathy. PLoS ONE 7:e34713
Chen M, Rose AE, Doudican N, Osman I, Orlow SJ (2009) Celastrol synergistically enhances temozolomide cytotoxicity in melanoma cells. Mol Cancer Res 7:1946–1953
Chen G, Zhang X, Zhao M, Wang Y, Cheng X, Wang D, Xu Y, Du Z, Yu X (2011) Celastrol targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent cytotoxicity in tumor cells. BMC Cancer 14(11):170
Cheng L, Smith DJ, Anderson RL, Nagley P (2011) Modulation of cellular Hsp72 levels in undifferentiated and neuron-like SH-SY5Y cells determines resistance to staurosporine-induced apoptosis. Biochem Biophys Res Commun 411:387–392
Cheung YT, Lau WK, Yu M, Lai CS, Yeung SC, So KF, Chang RC (2009) Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 30:127–135
Chiueh CC, Andoh T, Chock PB (2005) Induction of thioredoxin and mitochondrial survival proteins mediates preconditioning-induced cardioprotection and neuroprotection. Ann N Y Acad Sci 1042:403–418
Choi WS, Kruse SE, Palmiter RD, Xia Z (2008) Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci USA 105:15136–15141
Chou AP, Li S, Fitzmaurice AG, Bronstein JM (2010) Mechanisms of rotenone-induced proteasome inhibition. Neurotoxicology 31:367–372
Chow AM, Brown IR (2007) Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones 12:237–244
Cleren C, Calingasan NY, Chen J, Beal MF (2005) Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem 94:995–1004
Constantinescu R, Constantinescu AT, Reichmann H, Janetzky B (2007) Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. J Neural Transm Suppl 72:17–28
da Silva MM, Sartori A, Belisle E, Kowaltowski AJ (2003) Ischemic preconditioning inhibits mitochondrial respiration, increases H2O2 release, and enhances K+ transport. Am J Physiol Heart Circ Physiol 285:H154–H162
Dai Y, DeSano JT, Meng Y, Ji Q, Ljungman M, Lawrence TS, Xu L (2009) Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys 74:1217–1225
Dai Y, Desano J, Tang W, Meng X, Meng Y, Burstein E, Lawrence TS, Xu L (2010) Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB. PLoS ONE 5(12):e14153
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302:819–822
Domingues AF, Arduíno DM, Esteves AR, Swerdlow RH, Oliveira CR, Cardoso SM (2008) Mitochondria and ubiquitin-proteasomal system interplay: relevance to Parkinson’s disease. Free Radic Biol Med 45:820–825
Duty S, Jenner P (2011) Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 164:1357–1391
Dyllick-Brenzinger M, D’Souza CA, Dahlmann B, Kloetzel PM, Tandon A (2010) Reciprocal effects of alpha-synuclein overexpression and proteasome inhibition in neuronal cells and tissue. Neurotox Res 17:215–227
Elkon H, Melamed E, Offen D (2001) 6-Hydroxydopamine increases ubiquitin-conjugates and protein degradation: implications for the pathogenesis of Parkinson’s disease. Cell Mol Neurobiol 21:771–781
Faden AI, Stoica B (2007) Neuroprotection: challenges and opportunities. Arch Neurol 64:794–800
Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B (2009) Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci 10:109
Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, Chesselet MF (2004) Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol 187:418–429
Giordano S, Lee J, Darley-Usmar VM, Zhang J (2012) Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS ONE 7(9):e44610
Gosal D, Ross OA, Toft M (2006) Parkinson’s disease: the genetics of a heterogeneous disorder. Eur J Neurol 13:616–627
Greenamyre JT, Sherer TB, Betarbet R, Panov AV (2001) Complex I and Parkinson’s disease. IUBMB Life 52:135–141
Hajieva P, Mocko JB, Moosmann B, Behl C (2009) Novel imine antioxidants at low nanomolar concentrations protect dopaminergic cells from oxidative neurotoxicity. J Neurochem 110:118–132
Hauser RA (2009) New considerations in the medical management of early Parkinson’s disease: impact of recent clinical trials on treatment strategy. Parkinsonism Relat Disord 15(Suppl 3):S17–S21
Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur M (2002) Resistance of chemotherapy mediated by TrkB in neuroblastomas. Cancer Res 62:6462–6466
Höglinger GU, Carrard G, Michel PP, Medja F, Lombès A, Ruberg M, Friguet B, Hirsch EC (2003) Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson’s disease. J Neurochem 86:1297–1307
Hyun DH, Lee M, Halliwell B, Jenner P (2005) Effect of overexpression of wild-type or mutant parkin on the cellular response induced by toxic insults. J Neurosci Res 82:232–244
Imamura K, Takeshima T, Nakaso K, Ito S, Nakashima K (2008) Pramipexole has astrocyte-mediated neuroprotective effects against lactacystin toxicity. Neurosci Lett 440:97–102
Jantas D, Pytel M, Mozrzymas JW, Leskiewicz M, Regulska M, Antkiewicz-Michaluk L, Lason W (2008) The attenuating effect of memantine on staurosporine-, salsolinol- and doxorubicin-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurochem Int 52:864–877
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47:S161–S170
Jo H, Loison F, Hattori H, Silberstein LE, Yu H, Luo HR (2010) Natural product Celastrol destabilizes tubulin heterodimer and facilitates mitotic cell death triggered by microtubule-targeting anti-cancer drugs. PLoS ONE 5(4):e10318
Kalfon L, Youdim MB, Mandel SA (2007) Green tea polyphenol (−)-epigallocatechin-3-gallate promotes the rapid protein kinase C- and proteasome-mediated degradation of Bad: implications for neuroprotection. J Neurochem 100:992–1002
Kannaiyan R, Shanmugam MK, Sethi G (2011a) Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 303:9–20
Kannaiyan R, Manu KA, Chen L, Li F, Rajendran P, Subramaniam A, Lam P, Kumar AP, Sethi G (2011b) Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3K/Akt signaling pathways. Apoptosis 16:1028–1041
Kim Y, Kang H, Jang SW, Ko J (2011) Celastrol inhibits breast cancer cell invasion via suppression of NF-κB-mediated matrix metalloproteinase-9 expression. Cell Physiol Biochem 28:175–184
Kwon SJ, Ahn TB, Yoon MY, Jeon BS (2008) BV-2 stimulation by lactacystin results in a strong inflammatory reaction and apoptotic neuronal death in SH-SY5Y cells. Brain Res 1205:116–121
Lasorella A, Iavarone A, Israel MA (1995) Differentiation of neuroblastoma enhances Bcl-2 expression and induces alterations of apoptosis and drug resistance. Cancer Res 55:4711–4716
Layfield R, Cavey JR, Lowe J (2003) Role of ubiquitin-mediated proteolysis in the pathogenesis of neurodegenerative disorders. Ageing Res Rev 2:343–356
Lev N, Melamed E, Offen D (2006) Proteasomal inhibition hypersensitizes differentiated neuroblastoma cells to oxidative damage. Neurosci Lett 399:27–32
Levy OA, Malagelada C, Greene LA (2009) Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 14:478–500
Lombet A, Zujovic V, Kandouz M, Billardon C, Carvajal-Gonzalez S, Gompel A, Rostene W (2001) Resistance to induced apoptosis in the human neuroblastoma cell line SK-N-SH in relation to neuronal differentiation. Role of Bcl-2 protein family. Eur J Biochem 268:1352–1362
Lopes FM, Schröder R, da Frota ML, Zanotto-Filho A Jr, Müller CB, Pires AS, Meurer RT, Colpo GD, Gelain DP, Kapczinski F, Moreira JC, Fernandes Mda C, Klamt F (2010) Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res 1337:85–94
Luchtman DW, Song C (2010) Why SH-SY5Y cells should be differentiated. Neurotoxicology 31:164–165
Maruyama W, Naoi M (2002) Cell death in Parkinson’s disease. J Neurol 242:6–10
Maruyama W, Benedetti MS, Takahashi T, Naoi M (1997) A neurotoxin N-methyl(R)salsolinol induces apoptotic cell death in differentiated human dopaminergic neuroblastoma SH-SY5Y cells. Neurosci Lett 232:147–150
McNaught KS, Mytilineou C, Jnobaptiste R, Yabut J, Shashidharan P, Jennert P, Olanow CW (2002) Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J Neurochem 81:301–306
Middlemas DS, Kihl BK, Moody NM (1999) Brain derived neurotrophic factor protects human neuroblastoma cells from DNA damaging agents. J Neurooncol 45:27–36
Miloso M, Villa D, Crimi M, Galbiati S, Donzelli E, Nicolini G, Tredici G (2004) Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J Neurosci Res 75:241–252
Morita T (2010) Celastrol: a new therapeutic potential of traditional Chinese medicine. Am J Hypertens 23:821
Mytilineou C, McNaught KS, Shashidharan P, Yabut J, Baptiste RJ, Parnandi A, Olanow CW (2004) Inhibition of proteasome activity sensitizes dopamine neurons to protein alterations and oxidative stress. Neural Transm 111:1237–1251
Navarro A, Boveris A (2009) Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J Bioenerg Biomembr 41:517–521
Olanow CW (2007) The pathogenesis of cell death in Parkinson’s disease—2007. Mov Disord 22(Suppl 17):S335–S342
Olzmann JA, Chin LS (2008) Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy 4:85–87
Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W (2008a) Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis 32:16–25
Pan T, Zhu W, Zhao H, Deng H, Xie W, Jankovic J, Le W (2008b) Nurr1 deficiency predisposes to lactacystin-induced dopaminergic neuron injury in vitro and in vivo. Brain Res 1222:222–229
Perez-Alvarez S, Solesio ME, Manzanares J, Jordán J, Galindo MF (2009) Lactacystin requires reactive oxygen species and Bax redistribution to induce mitochondria-mediated cell death. Br J Pharmacol 158:1121–1130
Presgraves SP, Ahmed T, Borwege S, Joyce JN (2004) Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res 5:579–598
Rajendran P, Li F, Shanmugam MK, Kannaiyan R, Goh JN, Wong KF, Wang W, Khin E, Tergaonkar V, Kumar AP, Luk JM, Sethi G (2012) Celastrol suppresses growth and induces apoptosis of human hepatocellular carcinoma through the modulation of STAT3/JAK2 signaling cascade in vitro and in vivo. Cancer Prev Res (Phila) 5:631–643
Reaney SH, Johnston LC, Langston WJ, Di Monte DA (2006) Comparison of the neurotoxic effects of proteasomal inhibitors in primary mesencephalic cultures. Exp Neurol 202:434–440
Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW (2005) Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci 88:193–201
Rideout HJ, Lang-Rollin IC, Savalle M, Stefanis L (2005) Dopaminergic neurons in rat ventral midbrain cultures undergo selective apoptosis and form inclusions, but do not up-regulate iHSP70, following proteasomal inhibition. J Neurochem 93:1304–1313
Riepe MW, Ludolph AC (1997) Chemical preconditioning: a cytoprotective strategy. Mol Cell Biochem 174:249–254
Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE (2006) A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J 25:4808–4819
Schneider L, Giordano S, Zelickson BR, Johnson M S, A Benavides G, Ouyang X, Fineberg N, Darley-Usmar VM, Zhang J (2011) Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic Biol Med 51:2007–2017
Sgobbo P, Pacelli C, Grattagliano I, Villani G, Cocco T (2007) Carvedilol inhibits mitochondrial complex I and induces resistance to H2O2-mediated oxidative insult in H9C2 myocardial cells. Biochim Biophys Acta 1767:222–232
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (2003) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23:10756–10764
Suh J, Lee YA, Gwag BJ (2005) Induction and attenuation of neuronal apoptosis by proteasome inhibitors in murine cortical cell cultures. J Neurochem 95:684–694
Swerdlow RH (2009) Mitochondrial medicine and the neurodegenerative mitochondriopathies. Pharmaceuthicals 2:150–167
Tai HC, Schuman EM (2008) Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 9:826–838
Tai KK, Truong DD (2002) Activation of adenosine triphosphate-sensitive potassium channels confers protection against rotenone-induced cell death: therapeutic implications for Parkinson’s disease. J Neurosci Res 69:559–566
Terzioglu M, Galter D (2008) Parkinson’s disease: genetic versus toxin-induced rodent models. FEBS J 275:1384–1391
Tieu K, Zuon DM, Yu PH (1999) Differential effects of staurosporine and retinoic acid on the vulnerability of the SH-SY5Y neuroblastoma cells: involvement of bcl-2 and p53 proteins. J Neurosci Res 58:426–435
Walcott SE, Heikkila JJ (2010) Celastrol can inhibit proteasome activity and upregulate the expression of heat shock protein genes, hsp30 and hsp70, in Xenopus laevis A6 cells. Comp Biochem Physiol A Mol Integr Physiol 156:285–293
Wang WB, Feng LX, Yue QX, Wu WY, Guan SH, Jiang BH, Yang M, Liu X, Guo DA (2012) Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J Cell Physiol 227:2196–2206
Wenker SD, Chamorro ME, Vota DM, Callero MA, Vittori DC, Nesse AB (2010) Differential antiapoptotic effect of erythropoietin on undifferentiated and retinoic acid-differentiated SH-SY5Y cells. J Cell Biochem 110:151–161
Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI (2004) Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem 279:56053–56060
Whitworth AJ, Pallanck LJ (2009) The PINK1/Parkin pathway: a mitochondrial quality control system? J Bioenerg Biomembr 41:499–503
Wu J, Zhou Y, Wang L, Zuo J, Zhao W (2012) Terpenoids from root bark of Celastrus orbiculatus. Phytochemistry 75:159–168
Xun Z, Lee DY, Lim J, Canaria CA, Barnebey A, Yanonne SM, McMurray CT (2012) Retinoic acid-induced differentiation increases the rate of oxygen consumption and enhances the spare respiratory capacity of mitochondria in SH-SY5Y cells. Mech Ageing Dev 133:176–185
Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB (2010) Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins (Basel) 2:2428–2466
Yang H, Chen D, Cui QC, Yuan X, Dou QP (2006) Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res 66:4758–4765
Yang H, Landis-Piwowar KR, Chen D, Milacic V, Dou QP (2008) Natural compounds with proteasome inhibitory activity for cancer prevention and treatment. Curr Protein Pept Sci 9:227–239
Yong-Kee CJ, Salomonczyk D, Nash JE (2011) Development and validation of a screening assay for the evaluation of putative neuroprotective agents in the treatment of Parkinson’s disease. Neurotox Res 19:519–526
Yong-Kee CJ, Sidorova E, Hanif A, Perera G, Nash JE (2012a) Mitochondrial dysfunction precedes other sub-cellular abnormalities in an in vitro model linked with cell death in Parkinson’s disease. Neurotox Res 21:185–194
Yong-Kee CJ, Warre R, Monnier PP, Lozano AM, Nash JE (2012b) Evidence for synergism between cell death mechanisms in a cellular model of neurodegeneration in Parkinson’s disease. Neurotox Res 22:355–364
Acknowledgments
We kindly thank Ms. Barbara Korzeniak for her excellent technical assistance. This study was supported by Polish MNSW Scientific Network Fund No. 26/E-40/BWSN-0023/2008 and statutory funds for Institute of Pharmacology Polish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jantas, D., Roman, A., Kuśmierczyk, J. et al. The Extent of Neurodegeneration and Neuroprotection in Two Chemical In Vitro Models Related to Parkinson’s Disease is Critically Dependent on Cell Culture Conditions. Neurotox Res 24, 41–54 (2013). https://doi.org/10.1007/s12640-012-9374-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-012-9374-z