Abstract
Lead (Pb) accumulation, even in minute quantities, has adverse effects on the morphology, physiology, and biochemistry of almost all plants, resulting in various abnormalities. Silicon dioxide nanoparticles (SiO2-NPs) are used excessively to reduce abiotic stresses in a large variety of plant species. The present research work was designed to explore the role of SiO2-NPs in the mitigation of Pb toxicity in Ocimum basilicum. SiO2-NPs were green-synthesized from Arando donax plant extract. Characterization of green synthesized SiO2-NPs was assessed with UV-vs, XRD, FTIR, and SEM–EDS. To analyze the morphology and antioxidant enzyme activities in O. basilicum, 8 days old plants were subjected to 3 different concentrations of Pb and SiO2-NPs (50, 500, and 1000 ppm). Results of UV-vs, XRD, FTIR, and SEM–EDS showed the capping of SiO2-NPs by different functional groups (Si (CH3)3, and Si–O-Si) together with its crystalline structure. The average size of the nanoparticles was 26 nm which was confirmed by XRD analysis. Morphological analysis revealed that treatment with 500 ppm concentration of Pb resulted in a significant decrease in the length of root, shoot, and weight, in the ratio of 19, 14, and 10%, respectively. But treatment with 500 ppm (SiO2- NPs) significantly promoted root, shoot length, and weight of the plant, at the rate of 13, 22, and 7%, respectively. After the confirmation of ameliorative effect of SiO2-NPs, combined application of Pb + SiO2-NPs was tested. Root damage and Pb concentration in all the plant parts were much reduced. It was revealed that antioxidant activities of POD and APX were markedly decreased while those of the CAT and SOD increased. Results revealed that SiO2-NPs are an anti-stressor, that removes Pb from O. basilicum, by enhancing its antioxidant activity.
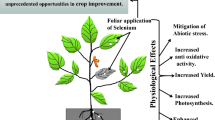
Graphical Abstract

Similar content being viewed by others
Data Availability
All the data generated or analyzed is present in this manuscript in the form of Tables and Figures. So, there is no extra data to present separately.
References
Nejad AR, Hatamian M, Kafi M, Souri MK, Shahbazi K (2018) Interaction of lead and nitrate on growth characteristics of ornamental judas tree (Cercis siliquastrum l.). Open Agric 3(1):670–677
Souri Mohammad Kazem, Hatamian Mansoure, Tesfamariam Tsehaye (2019) Plant growth stage influences heavy metal accumulation in leafy vegetables of garden cress and sweet basil. Chem Biol Technol Agric 6.1:1–7
Kumar A, Prasad MNV (2018) Plant-lead interactions: transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol Environ Saf 166:401–418
Youssef NA (2021) Changes in the morphological traits and the essential oil content of sweet basil (O. basilicum L.) as induced by cadmium and lead treatments. Int J Phytoremediation 23(3):291–299
Alfaraas AJ, Khairiah J, Ismail BS, Noraini T (2016) Effects of heavy metal exposure on the morphological and microscopical characteristics of the paddy plant. J Environ Biol 37(5):955
Minkina T, Fedorenko G, Nevidomskaya D, Fedorenko A, Chaplygin V, Mandzhieva S (2018) Morphological and anatomical changes of Phragmites australis Cav. due to the uptake and accumulation of heavy metals from polluted soils. Sci Total Environ 636:392–401
Fattahi B, Arzani K, Souri MK, Barzegar M (2019) Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimum basilicum L.). Ind Crop Prod 138:111584
Asadi S, Moghaddam M, GhasemiPirbalouti A, Fotovat A (2018) Evaluation of physiological characteristics and antioxidant activity of sweet basil (O. basilicum cv. Keshkeni luvelou) under different levels of methyl jasmonate and lead toxicity. J Iran Plant Ecophysiological Res 13(51):1–16
Dinu C, Vasile GG, Buleandra M, Popa DE, Gheorghe S, Ungureanu EM (2020) Translocation and accumulation of heavy metals in O. basilicum L. plants grown in a mining-contaminated soil. J Soils Sediments 20(4):2141–2154
Khalid N, Hussain M, Young HS, Ashraf M, Hameed M, Ahmad R (2018) Lead concentrations in soils and some wild plant species along two busy roads in Pakistan. Bull Environ Contam Toxicol 100(2):250–258
Al-Akeel K (2016) Lead uptake, accumulationand effects on plant growth of common reed (Pharagmites Australis (Cav.) Trin. ex Steudel) plants in hydroponic culture. Int J Adv Agric Environ Engg (IJAAEE) 3(2):391–394. https://doi.org/10.15242/IJAAEE.C1216047
Kohli SK, Handa N, Bali S, Khanna K, Arora S, Sharma A, Bhardwaj R (2019) Current scenario of Pb toxicity in plants: unraveling plethora of physiological responses. Rev Environ Contam Toxicol 249:153–197
Stratu A, Lobiuc A (2015) The influence of lead on seed germination and seedlings growth of Ocimum basilicum L. and Salvia coccinea Buchoz ex Etl. species. Analele Stiintifice ale Universitatii” Al I Cuza” din Iasi 61(1/2):39
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39(1):1–6
Singh M, Kumar J, Singh S, Singh VP, Prasad SM, Singh MPVVB (2015) Adaptation strategies of plants against heavy metal toxicity: a short review. Biochem Pharmacol (Los Angel) 4(161):2167–501
Liu D, Liu X, Chen Z, Xu H, Ding X (2010) Bioaccumulation of lead and the effects of lead on catalase activity, glutathione levels, and chlorophyll content in the leaves of wheat. Commun Soil Sci Plant Anal 41(8):935–944
Aydin SS, Büyük İ, Gündüzer EG, Büyük BP, Kandemir I, Cansaran-Duman D, Sümer ARAS (2016) Effects of Lead Pb and Cadmium Cd Elements on Lipid Peroxidation, Catalase Enzyme Activity and Catalase Gene Expression Profile in Tomato Plants. Journal of Agricultural Sciences 22(4):539–547
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria journal of medicine 54(4):287–293
Azarakhsh MR, Asrar Z, Mansouri H (2015) Effects of seed and vegetative stage cysteine treatments on oxidative stress response molecules and enzymes in Ocimum basilicum L. under cobalt stress. J Soil Sci Plant Nutrition 15(3):651–662
Balm ALT (2017) Protective effects of exogenous nitric oxide against lead toxicity in lemon balm (Melissa officinalis L.). Appl Ecol Environ Res 15(4):1605–1621
Romero-Puertas MC, Corpas FJ, Sandalio LM, Leterrier M, Rodríguez-Serrano M, Del Río LA, Palma JM (2006) Glutathione reductase from pea leaves: response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol 170(1):43–52
Kumar A, Prasad MNV, Sytar O (2012) Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 89(9):1056–1065
Ashkavand P, Tabari M, Zarafshar M, Tomásková I, Struve D (2015) Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leśne Prace Badawcze, 76(4):350–359. https://doi.org/10.1515/frp-2015-0034
Shah AA, Yasin NA, Akram K, Ahmad A, Khan WU, Akram W, Akbar M (2021) Ameliorative role of Bacillus subtilis FBL-10 and silicon against lead induced stress in Solanum melongena. Plant Physiol Biochem 158:486–496
Zargar SM, Mahajan R, Bhat JA, Nazir M, Deshmukh R (2019) Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system. 3 Biotech 9(3):1–16
Bharwana SA, Ali S, Farooq MA, Iqbal N, Abbas F, Ahmad MSA (2013) Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J Bioremed Biodeg 4(4):187
Araujo JDCTD, Nascimento CWAD, Cunha Filho FFD (2011) Disponibilidade de silício e biomassa de milho em solo contaminado por chumbo tratado com silicato. Ciência e Agrotecnologia 35(5):878–883
Sharma S, Singh VK, Kumar A, Mallubhotla S (2019) Effect of nanoparticles on oxidative damage and antioxidant defense system in plants. In: Roychoudhury A, Tripathi D (ed). John Wiley & Sons, Ltd, UK pp 315–333. https://doi.org/10.1002/9781119463665.ch17
Emamverdian A, Ding Y (2017) Effects of heavy metals’ toxicity on plants and enhancement of plant defense mechanisms of Si-mediation “Review. International Journal of Environmental and Agriculture Research (IJOEAR) 3(4):41–51
Chandra H, Kumari P, Bontempi E, Yadav S (2020) Medicinal plants: Treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatal Agric Biotechnol 24:101518
Gheshlaghpour J, Asghari B, Khademian R, Sedaghati B (2021) Silicon alleviates cadmium stress in basil (Ocimum basilicum L.) through alteration of phytochemical and physiological characteristics. Ind Crop Prod 163:113338
Imtiaz M, Rizwan MS, Mushtaq MA, Ashraf M, Shahzad SM, Yousaf B, ... and Tu S (2016) Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: a review. J Environ Manag, 183, 521-529
Krithiga N, Rajalakshmi A, Jayachitra A (2015) Green synthesis of silver nanoparticles using leaf extracts of clitoria ternatea and solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. J Nanosci p 1–8. https://doi.org/10.1155/2015/928204
Hossain Z, Mustafa G, Sakata K, Komatsu S (2016) Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. J Hazard Mater 304:291–305
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164(4):645–655
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M (2003) Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19(4):1357–1361
Jackson GC, Núñez JR (1964) Identification of silica present in the giant-reed (Arundo donax L.). J Agric Univ Puerto Rico 48(1):60–62
Setyawan N, Wulanawati A (2021) Yield and properties of nanobiosilica extracted from rice husk using technical grade chemicals: effect of extraction temperatures and times. In IOP conference series: Earth and environmental science 752(1):012036. https://doi.org/10.1088/1755-1315/752/1/012036
Pansuksan K, Sukprasert S, Karaket N (2020) Phytochemical compounds in Arundo donax L. Rhizome and antimicrobial activities. Pharmacogn J 12(2):287–292. https://doi.org/10.5530/pj.2020.12.45
Wang W, Martin JC, Zhang N, Ma C, Han A, Sun L (2011) Harvesting silica nanoparticles from rice husks. J Nanopart Res 13(12):6981–6990
Kumar V, Tiwari P, Krishnia L, Kumari R, Singh A, Ghosh A, Tyagi PK (2016) Green route synthesis of silicon/silicon oxide from bamboo. Adv Mater Lett 7(4):271–276
Athinarayanan J, Periasamy VS, Alhazmi M, Alatiah KA, Alshatwi AA (2015) Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram Int 41(1):275–281
Fahr M, Laplaze L, Bendaou N, Hocher V, El Mzibri M, Bogusz D, Smouni A (2013) Effect of lead on root growth. Front Plant Sci 4:175
Hussain A, Abbas N, Arshad F, Akram M, Khan ZI, Ahmad K, Mirzaei F (2013) Effects of diverse doses of Lead (Pb) on different growth attributes of Zea-Mays L. Agric Sci 4:262–265. https://doi.org/10.4236/as.2013.45037
Zheljazkov VD, Craker LE, Xing B (2006) Effects of Cd, Pb, and Cu on growth and essential oil contents in dill, peppermint, and basil. Environ Exp Bot 58(1–3):9–16
Amirmoradi S, Moghaddam, P. R., Koocheki, A., Danesh, S., and Fotovat, A. (2012) Effect of cadmium and lead on quantitative and essential oil traits of peppermint (Mentha piperita L.). Notulae Scientia Biologicae 4(4):101–109
Huang X, Duan S, Wu Q, Yu M, Shabala S (2020) Reducing cadmium accumulation in plants: structure–function relations and tissue-specific operation of transporters in the spotlight. Plants 9(2):223
Xia C, Hong L, Yang Y, Yanping X, Xing H, Gang D (2019) Protein changes in response to lead stress of lead-tolerant and lead-sensitive industrial hemp using swath technology. Genes 10(5):396
Ruiz E, Rodríguez L, Alonso-Azcárate J (2009) Effects of earthworms on metal uptake of heavy metals from polluted mine soils by different crop plants. Chemosphere 75(8):1035–1041
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PM (2016) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137
Khan ZS, Rizwan M, Hafeez M, Ali S, Adrees M, Qayyum MF, Sarwar MA (2020) Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ Sci Pollut Res 27(5):4958–4968
Cui J, Li Y, Jin Q, Li F (2020) Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ Sci Nano 7(1):162–171
Ding M, Zhu Q, Liang Y, Li J, Fan X, Yu X, ... Yu J (2017) Differential roles of three FgPLD genes in regulating development and pathogenicity in Fusarium graminearum. Fungal Genet Biol, 109, 46-52
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Al-Garni SM, Khan MMA, Bahieldin A (2019) Plant growth-promoting bacteria and silicon fertilizer enhance plant growth and salinity tolerance in Coriandrum sativum. Journal of Plant Interactions 14(1):386–396
Seregin IV, Shpigun LK, Ivanov VB (2004) Distribution and toxic effects of cadmium and lead on maize roots. Russ J Plant Physiol 51(4):525–533
Patel A, Pandey V, Patra DD (2015) Influence of tannery sludge on oil yield, metal uptake and antioxidant activities of Ocimum basilicum L. grown in two different soils. Ecol Eng 83:422–430
Chaudhary K, Jan S, Khan S (2016) Heavy metal ATPase (HMA2, HMA3, and HMA4) genes in hyperaccumulation mechanism of heavy metals. In: Ahmad P (ed). In plant metal interaction, Elsevier pp 545–556 . https://doi.org/10.1016/B978-0-12-803158-2.00023-0
Liang Y, Si J, Römheld V (2005) Silicon uptake and transport is an active process in Cucumis sativus. New Phytol 167(3):797–804
Greger M, Kabir AH, Landberg T, Maity PJ, Lindberg S (2016) Silicate reduces cadmium uptake into cells of wheat. Environ Pollut 211:90–97
Zhang C, Wang L, Nie Q, Zhang W, Zhang F (2008) Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ Exp Botany 62(3):300–307
Cuypers A, Hendrix S, Amaral dos Reis R, De Smet S, Deckers J, Gielen H, ... Keunen E (2016) Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front Plant Sci 7, 470
Elbaz A, Wei YY, Meng Q, Zheng Q, Yang ZM (2010) Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 19(7):1285–1293
Khademian R, Asghari B, Sedaghati B, Yaghoubian Y (2019) Plant beneficial rhizospheric microorganisms (PBRMs) mitigate deleterious effects of salinity in sesame (Sesamum indicum L.): Physio-biochemical properties, fatty acids composition and secondary metabolites content. Ind Crops Prod 136:129–139
Asghari B, Khademian R, Sedaghati B (2020) Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Scientia Horticulturae 263:109132
Jalloh MA, Chen J, Zhen F, Zhang G (2009) Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. J Hazard Mater 162(2–3):1081–1085
Sobrino-Plata J, Ortega-Villasante C, Flores-Cáceres ML, Escobar C, Del Campo FF, Hernández LE (2009) Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 77(7):946–954
Xuebin Q, Yatao X, Ahmad MI, Shehzad M, Zain M (2020) Silicon and its application methods improve physiological traits and antioxidants in Triticum aestivum (L.) under cadmium stress. J Soil Sci Plant Nutr 20(3):1110–1121
Geng A, Wang X, Wu L, Wang F, Wu Z, Yang H, Liu X (2018) Silicon improves growth and alleviates oxidative stress in rice seedlings (Oryza sativa L.) by strengthening antioxidant defense and enhancing protein metabolism under arsanilic acid exposure. Ecotoxicol Environ Saf 158:266–273
Acknowledgements
The authors thanks to National Center of Physics for XRD analysis and National Agricultural Research Center for providing Germplasm.
Funding
This work was supported by QAU-URF 2019.
Author information
Authors and Affiliations
Contributions
Hidayat Ullah: Formal analysis and investigation, Writing—Original Draft; Ilham Khan: Data curation, Writing—review and editing; Ghazala Mustafa *: Supervision, Conceptualization, review and editing; Murtaza Hasan: Methodology, Critical review; Junaid Shehzad: Software, Visualization; Sunbal Khalil Chaudhari: Resources, Critical review.
Corresponding author
Ethics declarations
Research Involving Humans/Animals
Not applicable.
Informed Consent
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent of Publication
All the Authors’ approved the final version to be published.
Competing Interest
The authors have no relevant financial or non-financial interests to disclose.
Conflicts of Interest
All the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ullah, H., Khan, I., Mustafa, G. et al. Molecular Characterization and Mitigative Role of Silicon Dioxide Nanoparticles in Ocimum Basilicum Under Lead (Pb) Stress. Silicon 15, 2551–2565 (2023). https://doi.org/10.1007/s12633-022-02178-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-02178-5