Abstract
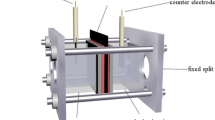
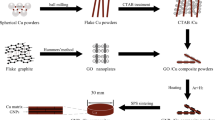
Cu-graphene (Gr) composite thin films were prepared by electrodeposition route using in-house synthesized Gr sheets. The Gr sheets were synthesized by the electrochemical exfoliation route using 1 M HClO4 acid as electrolyte. The Gr sheets were confirmed by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), field-emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM). The (002) plane of Gr sheets was observed at 2θ of 25.66°. The (002) plane confirmed the crystal structure of carbon peaks. The stretching vibration of C-C bond at a wavelength of 1577 cm−1 and other functional groups of carboxyl and epoxide groups were observed from FTIR. TEM confirmed the transparent structure of Gr sheets. The prepared Gr sheets were used as reinforcement at concentrations of 0.1 and 0.3 g/L with a copper matrix to synthesize the Cu-Gr composite. The prepared composite thin films were characterized by XRD, SEM, and energy-dispersion spectrometry (EDS) for morphological and analytical studies. The presence of Gr sheets in Cu-Gr composite was confirmed by EDS analysis. The prepared Cu-Gr nanocomposite thin film showed higher corrosion resistance compared with pure copper thin films in 3.5wt% NaCl, as confirmed by Tafel plots. Electrochemical impedance spectroscopy complimented the above results and showed that 0.3 g/L composite film achieved the highest film resistance.
Similar content being viewed by others
References
M.A. Amin and K.F. Khaled, Copper corrosion inhibition in O2-saturated H2SO4 solutions, Corros. Sci., 52(2010), No. 4, p. 1194.
K.F. Khaled, Studies of the corrosion inhibition of copper in sodium chloride solutions using chemical and electrochemical measurements, Mater. Chem. Phys., 125(2011), No. 3, p. 427.
I. Wlasny, P. Dabrowski, M. Rogala, I. Pasternak, W. Strupinski, J.M. Baranowski, and Z. Klusek, Impact of electrolyte intercalation on the corrosion of graphene-coated copper, Corros. Sci., 92(2015), p. 69.
R. Solmaz, E. Altunbaş Şahin, A. Döner, and G. Kardaş, The investigation of synergistic inhibition effect of rhodanine and iodide ion on the corrosion of copper in sulphuric acid solution, Corros. Sci., 53(2011), No. 10, p. 3231.
S.S. Chen, L. Brown, M. Levendorf, W.W. Cai, S.Y. Ju, J. Edgeworth, X.S. Li, C.W. Magnuson, A. Velamakanni, R.D. Piner, J.Y. Kang, J. Park, and R.S. Ruoff, Oxidation resistance of graphene-coated Cu and Cu/Ni alloy, ACS Nano, 5(2011), No. 2, p. 1321.
M.Z. Cai, D. Thorpe, D.H. Adamson, and H.C. Schniepp, Methods of graphite exfoliation, J. Mater. Chem., 22(2012), No. 48, p. 24992.
S.K. Sahoo and A. Mallik, Simple, fast and cost-effective electrochemical synthesis of few layer graphene nanosheets, Nano, 10(2015), No. 2, art. No. 1550019.
S.K. Sahoo and A. Mallik, Fundamentals of fascinating graphene nanosheets: A comprehensive study, Nano, 14(2019), No. 3, art. No. 1930003.
Y.H. Dong, Q.Q. Liu, and Q. Zhou, Corrosion behavior of Cu during graphene growth by CVD, Corros. Sci., 89(2014), p. 214.
M. Schriver, W. Regan, W.J. Gannett, A.M. Zaniewski, M.F. Crommie, and A. Zettl, Graphene as a long-term metal oxidation barrier: Worse than nothing, ACS Nano, 7(2013), No. 7, p. 5763.
F. Mohandes and M. Salavati-Niasari, Freeze-drying synthesis, characterization and in vitro bioactivity of chitosan/graphene oxide/hydroxyapatite nanocomposite, RSC Adv., 4(2014), No. 49, art. No. 25993.
H. Safardoust-Hojaghan and M. Salavati-Niasari, Degradation of methylene blue as a pollutant with N-doped graphene quantum dot/titanium dioxide nanocomposite, J. Clean. Prod., 148(2017), p. 31.
M. Mahdiani, F. Soofivand, F. Ansari, and M. Salavati-Niasari, Grafting of CuFe12O19 nanoparticles on CNT and graphene: Eco-friendly synthesis, characterization and photocatalytic activity, J. Clean. Prod., 176(2018), p. 1185.
H. Safajou, H. Khojasteh, M. Salavati-Niasari, and S. Mortazavi-Derazkola, Enhanced photocatalytic degradation of dyes over graphene/Pd/TiO2 nanocomposites: TiO2 nanowires versus TiO2 nanoparticles, J. Colloid Interface Sci., 498(2017), p. 423.
F. Tavakoli, M. Salavati-Niasari, A. Badiei, and F. Mohandes, Green synthesis and characterization of graphene nanosheets, Mater. Res. Bull., 63(2015), p. 51.
F. Mohandes and M. Salavati-Niasari, In vitro comparative study of pure hydroxyapatite nanorods and novel polyethylene glycol/graphene oxide/hydroxyapatite nanocomposite, J. Nanopart. Res., 16(2014), No. 9, art. No. 2604.
F. Soofivand and M. Salavati-Niasari, Co3O4/graphene nanocomposite: Pre-graphenization synthesis and photocatalytic investigation of various magnetic nanostructures, RSC Adv., 5(2015), No. 79, p. 64346.
H. Khojasteh, M. Salavati-Niasari, H. Safajou, and H. Safardoust-Hojaghan, Facile reduction of graphene using urea in solid phase and surface modification by N-doped graphene quantum dots for adsorption of organic dyes, Diamond Relat. Mater., 79(2017), p. 133.
H. Teymourinia, M. Salavati-Niasari, O. Amiri, and H. Safardoust-Hojaghan, Synthesis of graphene quantum dots from corn powder and their application in reduce charge recombination and increase free charge carriers, J. Mol. Liq., 242(2017), p. 447.
Z.L. Li, J. Zhao, J.L. Sun, F. Gong, and X.Y. Ni, Reinforcing effect of graphene on the mechanical properties of Al2O3/TiC ceramics, Int. J. Miner. Metall. Mater., 24(2017), No. 12, p. 1403.
X. Zeng, J. Teng, J.G. Yu, A.S. Tan, D.F. Fu, and H. Zhang, Fabrication of homogeneously dispersed graphene/Al composites by solution mixing and powder metallurgy, Int. J. Miner. Metall. Mater., 25(2018), No. 1, p. 102.
C.Y. Huang, S.P. Hu, and K. Chen, Influence of rolling temperature on the interfaces and mechanical performance of graphene-reinforced aluminum-matrix composites, Int. J. Miner. Metall. Mater., 26(2019), No. 6, p. 752.
W.M. Tian, S.M. Li, B. Wang, X. Chen, J.H. Liu, and M. Yu, Graphene-reinforced aluminum matrix composites prepared by spark plasma sintering, Int. J. Miner. Metall. Mater., 23(2016), No. 6, p. 723.
M. Pul, Effect of sintering temperature on pore ratio and mechanical properties of composite structure in nano graphene reinforced ZA27 based composites, Int. J. Miner. Metall. Mater., 27(2020), No. 2, p. 232.
M. Masjedi-Arani and M. Salavati-Niasari, Novel synthesis of Zn2GeO4/graphene nanocomposite for enhanced electrochemical hydrogen storage performance, Int. J. Hydrogen Energy, 42(2017), No. 27, p. 17184.
M. Masjedi-Arani and M. Salavati-Niasari, Ultrasonic assisted synthesis of a nano-sized Co2SnO4/graphene: A potential material for electrochemical hydrogen storage application, Int. J. Hydrogen Energy, 43(2018), No. 9, p. 4381.
M. Masjedi-Arani and M. Salavati-Niasari, Cd2SiO4/graphene nanocomposite: Ultrasonic assisted synthesis, characterization and electrochemical hydrogen storage application, Ultrason. Sonochem., 43(2018), p. 136.
M. Masjedi-Arani, M. Ghiyasiyan-Arani, O. Amiri, and M. Salavati-Niasari, CdSnO3-graphene nanocomposites: Ultrasonic synthesis using glucose as capping agent and characterization for electrochemical hydrogen storage, Ultrason. Sonochem., 61(2020), art. No. 104840.
A. Rose, K. Guru Prasad, T. Sakthivel, V. Gunasekaran, T. Maiyalagan, and T. Vijayakumar, Electrochemical analysis of graphene oxide/polyaniline/polyvinyl alcohol composite nanofibers for supercapacitor applications, Appl. Surf. Sci., 449(2018), p. 551.
M. Li, P. Xiong, F. Yan, S.J. Li, C.H. Ren, Z.C. Yin, A. Li, H.F. Li, X.M. Ji, Y.F. Zheng, and Y. Cheng, An overview of graphene-based hydroxyapatite composites for orthopedic applications, Bioact. Mater., 3(2018), No. 1, p. 1.
C.S. Wang, J.Y. Li, C. Amatore, Y. Chen, H. Jiang, and X.M. Wang, Gold nanoclusters and graphene nanocomposites for drug delivery and imaging of cancer cells, Angew. Chem. Int. Ed., 50(2011), No. 49, p. 11644.
A.K. Behera and A. Mallik, Ultrasound assisted electroplating of nano-composite thin film of Cu matrix with electrochemically in-house synthesized few layer graphene nano-sheets as reinforcement, J. Alloys Compd., 750(2018), p. 587.
Y. Raghupathy, A. Kamboj, M.Y. Rekha, N.P. Narasimha Rao, and C. Srivastava, Copper-graphene oxide composite coatings for corrosion protection of mild steel in 3.5wt% NaCl, Thin Solid Films, 636(2017), p. 107.
S.S. Li, G.S. Song, Q. Fu, and C.X. Pan, Preparation of Cugraphene coating via electroless plating for high mechanical property and corrosive resistance, J. Alloys Compd., 777(2019), p. 877.
G.X. Xie, M. Forslund, and J.S. Pan, Direct electrochemical synthesis of reduced graphene oxide (rGO)/copper composite films and their electrical/electroactive properties, ACS Appl. Mater. Interfaces, 6(2014), No. 10, p. 7444.
K. Chu, X.H. Wang, F. Wang, Y.B. Li, D.J. Huang, H. Liu, W.L. Ma, F.X. Liu, and H. Zhang, Largely enhanced thermal conductivity of graphene/copper composites with highly aligned graphene network, Carbon, 127(2018), p. 102.
Q. Zhang, Z.B. Qin, Q. Luo, Z. Wu, L. Liu, B. Shen, and W.B. Hu, Microstructure and nanoindentation behavior of Cu composites reinforced with graphene nanoplatelets by electroless co-deposition technique, Sci. Rep., 7(2017), art. No. 1338.
J. Wang, L.N. Guo, W.M. Lin, J. Chen, S. Zhang, S.D. Chen, T.T. Zhen, and Y.Y. Zhang, The effects of graphene content on the corrosion resistance, and electrical, thermal and mechanical properties of graphene/copper composites, New Carbon Mater., 34(2019), No. 2, p. 161.
F. Nazeer, Z. Ma, L.H. Gao, F.C. Wang, M.A. Khan, and A. Malik, Thermal and mechanical properties of copper-graphite and copper-reduced graphene oxide composites, Composites B, 163(2019), p. 77.
A.K. Behera, R. Chandran, S. Sarkar, and A. Mallik, An exploration on the use of in-house synthesized reduced few layer graphene particles as a reinforcement during sono-electroplating of Cu matrix composite films, J. Alloys Compd., 817(2020), art. No. 152713.
C.L.P. Pavithra, B.V. Sarada, K.V. Rajulapati, T.N. Rao, and G. Sundararajan, A new electrochemical approach for the synthesis of copper-graphene nanocomposite foils with high hardness, Sci. Rep., 4(2015), art. No. 4049.
Acknowledgements
The authors gratefully acknowledge the partial financial support of this work by the Department of Science and Technology (DST) India via grant number EEQ/2018/001452 and National Institute of Technology, Rourkela, India for the financial and infrastructure support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Behera, A.K., Das, A., Das, S. et al. Electrochemically functionalized graphene as an anti-corrosion reinforcement in Cu matrix composite thin films. Int J Miner Metall Mater 28, 1525–1533 (2021). https://doi.org/10.1007/s12613-020-2124-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-020-2124-y