Abstract
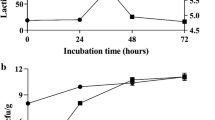
Herein, we explore the probiotic potentials and soybean meal (SBM) compound feed fermentative applications of Lactobacillus plantarum SK1305 strain isolated previously from Korean green chili pickled pepper (gochu-jangajji). The isolate exhibited higher acid (pH 2.5) and bile tolerance (0.3%, w/v) up to 2 h and 4 h, respectively. The cell-free culture supernatant (CFCS) displayed a broad spectrum antibacterial activities against various pathogens, which may be ascribed to high lactic acid production (L-form, 86.8 ± 0.8 mM and D-form, 44.8 ± 0.2 mM). Further, the strain displayed high cell-surface hydrophobicity (92.7 ± 1.0%), coupled with low auto-aggregation (23.6 ± 4.4%) but relatively higher co-aggregation properties with C. perfringens (49.6 ± 0.6%) as well as H2O2 (1.0 mM) resistant property. Additionally, the isolate displayed significant DPPH free radical scavenging activity (55.2 ± 0.6%) and superoxide reducing ability in MAC-T cells. Considering safety, the isolate has no transmissible antibiotic resistant genes and harmful enzymes as well as non-hemolytic activities. Ushered by these appreciable probiotic properties, the isolate was used for solid state fermentation (SSF) of SBM compound feed. Notably, we observed a higher strain adaptability (> 1010 CFU/g) following the production of L- (> 6.0 ± 0.0 mM) and D-form (> 5.2 ± 0.3 mM) lactic acid during fermentation for 8 h. The methanolic extracts of fermented feed displayed high antibacterial and antioxidant activities, affirming the potential functional activities of fermented compound feeds. Therefore, L. plantarum SK1305 may act as a worthy inoculum toward fermentation of feed with enhanced nutritional properties.





Similar content being viewed by others
References
Wang J, Han M, Zhang G, Qiao S, Li D, Ma X (2016a) The signal pathway of antibiotic alternatives on intestinal microbiota and immune function. Curr Protein Pept Sci 17:785–796
Hossain MI, Sadekuzzaman M, Ha SD (2017) Probiotics as potential alternative biocontrol agents in the agriculture and food industries: a review. Food Res Int 100:63–73
FAO/WHO (2006) Probiotics in food. Health and nutritional properties and guidelines for evaluation in FAO food and nutrition paper. No. 85. WHO/FAO, Rome
Kanmani P, Satish Kumar R, Yuvaraj N, Paari KA, Pattukumar V, Arul V (2013) Probiotics and its functionally valuable products—a review. Crit Rev Food Sci Nutr 53:641–658
Wang L, Zhang H, Rehman MU, Mehmood K, Jiang X, Iqbal M, Tong X, Li J (2018) Antibacterial activity of Lactobacillus plantarum isolated from Tibetan yaks. Microb Pathog 115:293–298
Body Ecology (2009) Lactobacillus Plantarum: The Key Benefits of This “Superstar” Probiotic and How to Get It in Your Diet. https://bodyecology.com/articles/lactobacillus_plantarum_benefits.php
Argyri AA, Zoumpopoulou G, Karatzas KAG, Tsakalidou E, Nychas GJE, Panagou EZ, Tassou CC (2013) Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol 33:282–291
Patra JK, Das G, Paramithiotis S, Shin HS (2016) Kimchi and other widely consumed traditional fermented foods of Korea: a review. Front Microbiol 7:1–15
Son SH, Jeon HL, Jeon EB, Lee NK, Park YS, Kang DK, Paik HD (2017) Potential probiotic Lactobacillus plantarum Ln4 from kimchi: evaluation of β-galactosidase and antioxidant activities. LWT Food Sci Technol 85:181–186
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63:3615–3626
Canibe N, Jensen BB (2003) Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J Anim Sci 81:2019–2031
Engberg RM, Hammershoj M, Johansen NF, Abousekken MS, Steenfeldt S, Jensen BB (2009) Fermented feed for laying hens: effects on egg production, egg quality, plumage condition and composition and activity of the intestinal microflora. Br Poult Sci 50:228–239
Shrivastava B, Jain KK, Kalra A, Kuhad RC (2014) Bioprocessing of wheat straw into nutritionally rich and digested cattle feed. Sci Rep 4:1–9
Seo SH, Cho SJ (2016) Changes in allergenic and antinutritional protein profiles of soybean meal during solid-state fermentation with Bacillus subtilis. LWT Food Sci Technol 70:208–212
Zhu J, Gao M, Zhang R, Sun Z, Wang C, Yang F, Huang T, Qu S, Zhao L, Li Y, Hao Z (2017) Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb Cell Factories 16:1–10
Adeyemo SM, Onilude AA (2013) Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Nigerian Food J 31:84–90
Wang L, Zhou H, He R, Xu W, Mai K, He G (2016b) Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 464:87–94
Song YS, Pérez VG, Pettigrew JE, Martinez-Villaluenga C, de Mejia EG (2010) Fermentation of soybean meal and its inclusion in diets for newly weaned pigs reduced diarrhea and measures of immunoreactivity in the plasma. Anim Feed Sci Technol 159:41–49
Coghetto CC, Vasconcelos CB, Brinques GB, Ayub MAZ (2016) Lactobacillus plantarum BL011 cultivation in industrial isolated soybean protein acid residue. Braz J Microbiol 47:941–948
Hernandez D, Cardell E, Zarate V (2005) Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J Appl Microbiol 99:77–84
Kim PI, Jung MY, Chang YH, Kim S, Kim SJ, Park YH (2007) Probiotic properties of Lactobacillus and Bifidobacterium strains isolated from porcine gastrointestinal tract. Appl Microbiol Biotechnol 74:1103–1111
Bao Y, Zhang Y, Zhang Y, Liu Y, Wang S, Dong X, Wang Y, Zhang H (2010) Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 21:695–701
Kheadr E, Dabour N, Le Lay C, Lacroix C, Fliss I (2007) Antibiotic susceptibility profile of Bifidobacteria as affected by oxgall, acid, and hydrogen peroxide stress. Antimicrob Agents Chemother 51:169–174
Xu H, Jeong HS, Lee HY, Ahn J (2009) Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett Appl Microbiol 49:434–442
Kos B, Suskovic J, Vukovic S, Simpraga M, Frece J, Matosic S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M29. J Appl Microbiol 94:981–987
Del Re B, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, auto-aggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31:438–442
Li S, Zhao Y, Zhang L, Zhang X, Huang L, Li D, Niu C, Yang Z, Wang Q (2012) Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem 135:1914–1919
Kim DO, Lee KW, Lee HJ, Lee CY (2002) Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem 50:3713–3717
Han SG, Newsome B, Hennig B (2013) Titanium dioxide nanoparticles increase inflammatory responses in vascular endothelial cells. Toxicology 306:1–8
Angmo K, Kumari A, Bhalla TC (2016) Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci Technol 66:428–435
Sanders ME, Huis J (1999) Bringing a probiotic-containing functional food to the market: microbiological, product, regulatory and labeling issues. Antonie Van Leeuwenhoek 76:293–315
Trontel A, Batušić A, Gusić I, Slavica A, Šantek B, Novak S (2011) Production of d-and l-lactic acid by mono-and mixed cultures of Lactobacillus sp. Food Technol Biotechnol 49:75–82
Gautam N, Sharma N (2015) Evaluation of probiotic potential of new bacterial strain, Lactobacillus spicheri G2 isolated from Gundruk. Proc Natl Acad Sci India B Biol Sci 85:979–986
Vijayakumar M, Ilavenil S, Kim DH, Arasu MV, Priya K, Choi KC (2015) In vitro assessment of the probiotic potential of Lactobacillus plantarum KCC-24 isolated from Italian rye-grass (Lolium multiflorum) forage. Anaerobe 32:90–97
Saelim K, Jampaphaeng K, Maneerat S (2017) Functional properties of Lactobacillus plantarum S0/7 isolated fermented stinky bean (Sa Taw Dong) and its use as a starter culture. J Funct Foods 38:370–377
Schnürer J, Magnusson J (2005) Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Technol 16:70–78
Boke H, Aslim B, Alp G (2010) The role of resistance to bile salts and acid tolerance of exopolysaccharides (EPSS) produced by yogurt starter bacteria. Arch Biol Sci 62:323–328
Goh YJ, Klaenhammer TR (2010) Functional roles of aggregation-promoting-like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl Environ Microbiol 76:5005–5012
Rajoka MSR, Mehwish HM, Siddiq M, Haobin Z, Zhu J, Yan L, Shao D, Xu X, Shi J (2017) Identification, characterization, and probiotic potential of Lactobacillus rhamnosus isolated from human milk. LWT Food Sci Technol 84:271–280
Missotten JAM, Goris J, Michiels J, Van Coillie E, Herman L, De Smet S, Dierick NA, Heyndrickx M (2009) Screening of isolated lactic acid bacteria as potential beneficial strains for fermented liquid pig feed production. Anim Feed Sci Technol 150:122–138
Dias FS, Duarte WF, Schwan RF (2013) Evaluation of adhesive properties of presumptive probiotic Lactobacillus plantarum strains. Biosci J 29:1678–1686
Tareb R, Bernardeau M, Gueguen M, Vernoux JP (2013) In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J Med Microbiol 62:637–649
Das D, Goyal A (2015) Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT Food Sci Technol 61:263–268
Yang X, Li L, Duan Y, Yang X (2017) Antioxidant activity of Lactobacillus plantarum JM113 in vitro and its protective effect on broiler chickens challenged with deoxynivalenol. J Anim Sci 95:837–846
Park SY, Lim SD (2015) Probiotic characteristics of Lactobacillus plantarum FH185 isolated from human feces. Korean J Food Sci Anim Resour 35:615–621
De Verse M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J (2003) Probiotics-compensation for lactose insufficiency. Am J Clin Nutr 73:421–429
Humblot C, Murkovic M, Rigottier-Gois L, Bensaada M, Bouclet A, Andrieux C, Anba J, Rabot S (2007) β-glucuronidase in human intestinal microbiota is necessary for the colonic genotoxicity of the food-borne carcinogen 2-amino-3-methylimidazo [4, 5-f] quinoline in rats. Carcinogenesis 28:2419–2425
Danielsen M, Wind A (2003) Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol 82:1–11
Gueimonde M, Sánchez B, de los Reyes-Gavilán CG, Margolles A (2013) Antibiotic resistance in probiotic bacteria. Front Microbiol 4:1–6
Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, Valtonen V (2003) Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 36:775–780
Dujardin M, Elain A, Lendormi T, Le Fellic M, Le Treut Y, Sire O (2014) Keeping under control a liquid feed fermentation process for pigs: a reality scale pilot based study. Anim Feed Sci Technol 194:81–88
Gan RY, Shah NP, Wang MF, Lui WY, Corke H (2017) Lactobacillus plantarum WCFS1 fermentation differentially affects antioxidant capacity and polyphenol content in mung bean (Vigna radiata) and soya bean (Glycine max) milks. J Food Process Preserv 41:1–9
Choct M, Dersjant-Li Y, McLeish J, Peisker M (2010) Soy oligosaccharides and soluble non-starch polysaccharides: a review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian Australas J Anim Sci 23:1386–1398
Singh BP, Vij S (2018) α-Galactosidase activity and oligosaccharides reduction pattern of indigenous lactobacilli during fermentation of soy milk. Food Biosci 22:32–37
Olstorpe M, Lyberg K, Lindberg JE, Schnürer J, Passoth V (2008) Population diversity of yeasts and lactic acid bacteria in pig feed fermented with whey, wet wheat distillers’ grains, or water at different temperatures. Appl Environ Microbiol 74:1696–1703
Merrell DS, Camilli A (2002) Acid tolerance of gastrointestinal pathogens. Curr Opin Microbiol 5:51–55
van Winsen RL, Urlings BA, Lipman LJ, Snijders JM, Keuzenkamp D, Verheijden JH, van Knapen F (2001) Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl Environ Microbiol 67:3071–3076
Missotten JAM, Michiels J, Goris J, Herman L, Heyndrickx M, De Smet S, Dierick NA (2007) Screening of two probiotic products for use in fermented liquid feed. Livest Sci 108:232–235
Zhang Y, Vadlani PV, Kumar A, Hardwidge PR, Govind R, Tanaka T, Kondo A (2016) Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl Microbiol Biotechnol 100:279–288
Zhao D, Shah NP (2014) Changes in antioxidant capacity, isoflavone profile, phenolic and vitamin contents in soymilk during extended fermentation. LWT Food Sci Technol 58:454–462
Chandra P, Arora DS (2016) Production of antioxidant bioactive phenolic compounds by solid-state fermentation on agro-residues using various fungi isolated from soil. Asian J Biotechnol 8:8–15
Wang NF, Le GW, Shi YH, Zeng Y (2014) Production of bioactive peptides from soybean meal by solid state fermentation with lactic acid bacteria and protease. Adv J Food Sci Technol 6:1080–1085
Sanjukta S, Rai AK, Muhammed A, Jeyaram K, Talukdar NC (2015) Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation. J Funct Foods 14:650–658
Chi CH, Cho SJ (2016) Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT Food Sci Technol 68:619–625
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No.312058–03) and Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ010906), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Kai-Min Niu and Damini Kothari are the co-first authors.
Rights and permissions
About this article
Cite this article
Niu, KM., Kothari, D., Cho, SB. et al. Exploring the Probiotic and Compound Feed Fermentative Applications of Lactobacillus plantarum SK1305 Isolated from Korean Green Chili Pickled Pepper. Probiotics & Antimicro. Prot. 11, 801–812 (2019). https://doi.org/10.1007/s12602-018-9447-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9447-2