Abstract
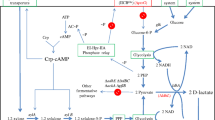
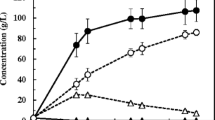
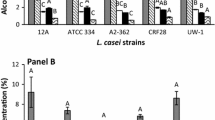
D-lactic acid is used as a monomer in the production of poly-D-lactic acid (PDLA), which is used to form heat-resistant stereocomplex poly-lactic acid. To produce cost-effective D-lactic acid by using all sugars derived from biomass efficiently, xylose-assimilating genes encoding xylose isomerase and xylulokinase were cloned into an L-lactate-deficient strain, Lactobacillus plantarum. The resulting recombinant strain, namely L. plantarum NCIMB 8826 ∆ldhL1-pLEM-xylAB, was able to produce D-lactic acid (at optical purity >99 %) from xylose at a yield of 0.53 g g−1. Simultaneous utilization of glucose and xylose to produce D-lactic acid was also achieved by this strain, and 47.2 g L−1 of D-lactic acid was produced from 37.5 g L−1 glucose and 19.7 g L−1 xylose. Corn stover and soybean meal extract (SBME) were evaluated as cost-effective medium components for D-lactic acid production. Optimization of medium composition using response surface methodology resulted in 30 % reduction in enzyme loading and 70 % reduction in peptone concentration. In addition, we successfully demonstrated D-lactic acid fermentation from corn stover and SBME in a fed-batch fermentation, which yielded 61.4 g L−1 D-lactic acid with an overall yield of 0.77 g g−1. All these approaches are geared to attaining high D-lactic acid production from biomass sugars to produce low-cost, highly thermostable biodegradable plastics.



Similar content being viewed by others
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902. doi:10.1016/j.biotechadv.213.04.002
Ahmed FE (2006) Genetically modified probiotics. In: Goktepe I, Juneja VK, Ahmedna M (eds) Probiotics in food safety and human health. CRC Press, FL, pp. 229–250
Batal AB, Douglas MW, Engram AE, Parsons CM (2000) Protein dispersibility index as an indicator of adequately processed soybean meal. Poult Sci 79:1592–1596. doi:10.1093/ps/79.11.1592
Bustos G, Moldes AB, Cruz JM, Domínguez JM (2005) Influence of the metabolism pathway on lactic acid production from hemicellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol Prog 21:793–798. doi:10.1021/bp049603v
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475. doi:10.1080/00401706.1960.10489912
Derré I, Rapoport G, Msadek T (1999) CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in Gram-positive bacteria. Mol Microbiol 31:117–131. doi:10.1046/j.1365-2958.1999.01152.x
Fons M, Hégé T, Ladiré M, Raibaud P, Ducluzeau R, Maguin E (1997) Isolation and characterization of a plasmid from Lactobacillus fermentum conferring erythromycin resistance. Plasmid 37:199–203. doi:10.1006/plas.1997.1290
Guragain YN, Wilson J, Staggenborg S, McKinney L, Wang D, Vadlani PV (2013) Evaluation of pelleting as a pre-processing step for effective biomass deconstruction and fermentation. Biochem Eng J 77:198–207. doi:10.1016/j.bej.2013.05.014
Guo W, He R, Ma L, Jia W, Li D, Chen S (2014) Construction of a constitutively expressed homo-fermentative pathway in Lactobacillus brevis. Appl Microbiol Biotechnol 98:6641–6650. doi:10.1007/s00253-014-5703-x
Helanto M, Kiviharju K, Leisola M, Nyyssoelae A (2007) Metabolic engineering of Lactobacillus plantarum for production of L-ribulose. Appl Environ Microbiol 73:7083–7091. doi:10.1128/AEM.01180-07
Ishida N, Suzuki T, Tkuhiro K, Nagamori E, Onishi T, Saitoh S, Kitamoto K, Takahashi H (2006) D-lactic acid production by metabolically engineered Saccharomyces cerevisiae. J Biosic Bioeng 101:172–177. doi:10.1263/jbb.101.172
Kwon S, Lee PC, Lee EG, Chang YK, Chang N (2000) Production of lactic acid by Lactobacillus rhamnosus with vitamin-supplemented soybean hydrolysate. Enzym Microb Technol 26:209–215. doi:10.1016/S0141-0229(99)00134-9
Kandler O (1983) Carbohydrate metabolism in lactic acid bacteria. A Van Leeuw J Microb 49:209–224. doi:10.1007/BF00399499
Kashket ER (1987) Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol Rev 46:233–244. doi:10.1111/j.1574-6968.1987.tb02463.x
Li Z, Ding S, Li Z, Tan T (2006) L-lactic acid production by Lactobacillus casei fermentation with corn steep liquor-supplemented acid-hydrolysate of soybean meal. Biotechnol J 1:1453–1458. doi:10.1002/biot.200600099
Lazazzera BA, Grossman AD (1997) A regulatory switch involving a Clp ATPase. BioEssays 19:455–458. doi:10.1002/bies.950190604
Lokman BC, Heerikhuisen M, Leer RJ, van den Broek A, Borsboom Y, Chaillou S, Postma P, Pouwels PH (1997) Regulation of expression of the Lactobacillus pentosus xylAB operon. J Bacteriol 179:5391–5397
Liu GQ, Wang XL (2007) Optimization of critical medium components using response surface methodology for biomass and extracellular polysaccharide production by Agaricus blazei. Appl Microbiol Biotechnol 74:78–83. doi:10.1007/s00253-006-0661-6
Li Y, Ruan R, Chen P, Liu Z, Pan X, Lin X, Liu Y, Mok C, Yang T (2004) Enzymatic hydrolysis of corn stover pretreated by combined dilute alkaline treatment and homogenization. Trans ASAE 47:821–825
Li C, Tao F, Ni J, Wang Y, Yao F, Xu P (2015) Enhancing the light-driven production of D-lactate by engineering cyanobacterium using a combinational strategy. Sci Rep. doi:10.1038/srep0977
Maiti B, Rathore A, Srivastava S, Shekhawat M, Srivastava P (2011) Optimization of process parameters for ethanol production from sugar cane molasses by Zymomonas mobilis using response surface methodology and genetic algorithm. Appl Microbiol Biotechnol 90:385–395. doi:10.1007/s00253-011-3158-x
McCracken A, Turner MS, Giffard P, Hafner LM, Timms P (2000) Analysis of promoter sequences from Lactobacillus and Lactococcus and their activity in several Lactobacillus species. Arch Microbiol 173:383–389. doi:10.1007/s002030000159
Narita J, Okano K, Kitao T, Ishida S, Sewaki T, Sung MH, Fukuda H, Kondo A (2006) Display of alpha-amylase on the surface of Lactobacillus casei cells by use of the PgsA anchor protein, and production of lactic acid from starch. Appl Environ Microbiol 72:269–275. doi:10.1128/AEM.72.1.269-275.2006
Okano K, Yoshida S, Tanaka T, Ogino C, Fukuda H, Kondo A (2009a) Homo-D-lactic acid fermentation from arabinose by redirection of the phosphoketolase pathway to the pentose phosphate pathway in L-Lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75:5175–5178. doi:10.1128/AEM.00573-09
Okano K, Yoshida S, Yamada R, Tanaka T, Ogino C, Fukuda H, Kondo A (2009b) Improved production of homo-D-lactic acid via xylose fermentation by introduction of xylose assimilation genes and redirection of the phosphoketolase pathway to the pentose phosphate pathway in L-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75:7858–7861. doi:10.1128/AEM.01692-09
Okano K, Zhang Q, Shinkawa S, Yoshida S, Tanaka T, Fukuda H, Kondo A (2009c) Efficient production of optically pure D-lactic acid from raw corn starch by using a genetically modified L-lactate dehydrogenase gene-deficient and alpha-amylase-secreting Lactobacillus plantarum strain. Appl Environ Microbiol 75:462–467. doi:10.1128/AEM.01514-08
Okano K, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85:413–423. doi:10.1007/s00253-009-2280-5
Piard JC, Desmazeaud M (1991) Inhibiting factors produced by lactic acid bacteria. 1. Oxygen metabolites and catabolism end-products. Lait 71(5):525–541. doi:10.1051/lait:1991541
Pollack MA, Lindner M (1942) Glutamine and glutamic acid as growth factors for lactic acid bacteria. J Biol Chem 143:655–661
Posno M, Leer RJ, van Luijk N, van Giezen MJF, Heuvelmans PTHM, Lokman BC, Pouwels PH (1991a) Incompatibility of Lactobacillus plasmids and segregational instability of the introduced vectors. Appl Environ Microbiol 57:1822–1828
Posno M, Heuvelmans PTH, van Giezen MJF, Lokman BC (1991b) Complementation of the inability of Lactobacillus strains to utilize D-xylose with D-xylose catabolism-encoding genes. Appl Environ Microbiol 57:2764–2766
Rixon JE, Warner PJ (2003) Introduction, background, relevant genetic techniques and terms. In: BJB W, Warner PJ (eds) Genetics of lactic acid bacteria. Plenum Publisher, New York, pp. pp 1–pp24
Rochat T, Gratadoux JJ, Gruss A, Corthier G, Maguin E, Langella P, Van de guchte M (2006) Production of a heterologous nonheme catalase by Lactobacillus casei: an efficient tool for removal of H2O2 and protection of Lactobacillus bulgaricus from oxidative stress in milk. Appl Environ Microbiol 72:5143–5149. doi:10.1128/AEM.00482-06
Serror P, Sasaki T, Ehrlich S, Maguin E (2002) Electrotransformation of Lactobacillus delbrueckii subsp bulgaricus and L. delbrueckii subsp lactis with various plasmids. Appl Environ Microbiol 68:46–52. doi:10.1128/AEM.68.1.46-52.2002
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Wang L, Zhao B, Li F, Xu K, Ma C, Tao F, Li Q, Xu P (2011) Highly efficient production of D-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl Microbiol Biotechnol 89:1009–1017. doi:10.1128/genomeA.00465-14
Yoshida S, Okano K, Tanaka T, Ogino C, Kondo A (2011) Homo-D-lactic acid production from mixed sugars using xylose-assimilating operon-integrated Lactobacillus plantarum. Appl Microbiol Biotechnol 92:67–76. doi:10.1007/s00253-011-3356-6
Yadav AK, Chaudhari AB, Kothari RM (2011) Bioconversion of renewable resources into lactic acid: an industrial view. Crit Rev Biotechnol 31:1–19. doi:10.3109/07388550903420970
Yanez R, Moldes AB, Alonso JL, Parajo JC (2003) Production of D-lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp. torquens. Biotechnol Lett 25:1161–1164. doi:10.1023/A:1024534106483
Zambare VP, Christopher LP (2012) Optimization of enzymatic hydrolysis of corn stover for improved ethanol production. Energy Explor Exploit 30:193–205. doi:10.1186/1754-6834-6-171
Zhang Y, Kumar A, Vadlani PV, Narayanan S (2013) Production of nitrogen-based platform chemical: cyanophycin biosynthesis using recombinant Escherichia coli and renewable media substitutes. J Chem Tech Biot 88:1321–1327. doi:10.1002/jctb.3978
Zhang Y, Vadlani PV (2013) D-lactic acid biosynthesis from biomass-derived sugars via Lactobacillus delbrueckii fermentation. Bioprocess Biosyst Eng 36:1897–1904. doi:10.1007/s00449-013-0965-8
Zhang Y, Vadlani PV (2015) Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. BioSci Bioeng 119:694–699. doi:10.1016/j.jbiosc.2014.10.027
Zhu Y, Lee YY, Elander RT (2007) Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and cofermentation. Appl Biochem Biotechnol 137:721–738. doi:10.1007/s12010-007-9092-9
Acknowledgments
The authors thank Dr. Serror for donating the pLEM415 plasmid. The authors also appreciate Novozymes Inc. for donating enzymes. This work was supported by the Consortium for Plant Biotechnology Research (CPBR) and the Gary and Betty Lortscher Endowment. This is contribution no. 15-350-J from the Kansas Agricultural Experiment Station.
Ethical statement
No human subject or animal research was involved in this study. Further, no endangered or protected species were used in the study.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 270 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Vadlani, P.V., Kumar, A. et al. Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum . Appl Microbiol Biotechnol 100, 279–288 (2016). https://doi.org/10.1007/s00253-015-7016-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7016-0