Abstract
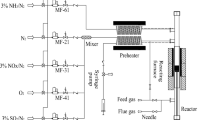
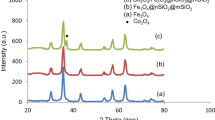
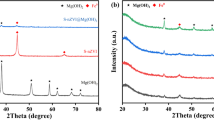
The recovery of heterogeneous catalysts can save costs and avoid secondary pollution, but its separation efficiency and recovery cost are limited by conventional separation methods such as precipitation–flocculation, centrifugation and filtration. In this paper, we found that surface-defective metal sulfides/oxides (WS2, CuS, ZnS, MoS2, CdS, TiO2, MoO2 and ZnO) commonly used in advanced oxidation processes (AOPs) could be magnetically recovered at room temperature and atmospheric pressure by mechanically mixing with Fe3O4. Zeta potential, Raman, X-ray photoelectron spectroscopy (XPS) and electro-spin resonance (ESR) spectra were measured to explore the mechanism of the magnetic separation phenomenon. The exposed active metal sites on the surface of defective metal sulfides/oxides are beneficial for the formation of chemical bonds, which are combined with electrostatic force to be responsible for the magnetic separation. Moreover, other factors affecting the magnetic separation were also investigated, such as the addition of amount of Fe3O4, different solvents and particle sizes. Finally, WS2 was chosen to be applied as a co-catalyst in Fenton reaction, which could be well separated by the magnetic Fe3O4 to achieve the recycle of catalyst in Fenton reaction. Our research provides a general strategy for the recycle of metal sulfides/oxides in the catalytic applications.







Similar content being viewed by others
References
Zea H, Lester K, Datye AK, Rightor E, Gulotty R, Waterman W, Smith M. The influence of Pd–Ag catalyst restructuring on the activation energy for ethylene hydrogenation in ethylene–acetylene mixtures. Appl Catal A-Gen. 2005;282(1):237.
Cheng Z, Wang W, Yang LM, Xu Z, Ji ZG, Huang ST. Preparation of La-TiO2 and photocatalytic degradation of petrochemical secondary effluent. Chin. J. Rare Meals. 2018;42(9):950.
Jiang Z, Zhu K, Lin Z, Jin S, Guang L. Structure and Raman scattering of Mg-doped ZnO nanoparticles prepared by sol–gel method. Rare Met. 2018;37(10):881.
Zhao D, Liao Y, Zhang Z. Toxicity of ionic liquids. Clean. 2007;35(1):42.
Carl Christoph T, Christian M, Willi B, Sebastian R, André H, Rainer H. Modern separation techniques for the efficient workup in organic synthesis. Angew Chem Int Ed. 2010;41(21):3964.
Barbaro P, Liguori F, Linares N, Marrodan CM. Heterogeneous bifunctional metal/acid catalysts for selective chemical processes. Eur J Inorg Chem. 2012;2012(24):3807.
Anipsitakis GP, Dionysiou DD. Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol. 2004;38(13):3705.
Nadkarni SV, Gawande MB, Jayaram RV, Nagarkar JM. Synthesis of bis(indolyl)methanes catalyzed by surface modified zirconia. Catal Commun. 2008;9(8):1728.
Fang W, Zhou L, Shen B, Zhou Y, Yi Q, Xing M, Zhang J. Advanced visible-light-driven activity for the degradation of organic dyes. Res Chem Intermed. 2018;44(8):4609.
Liu Q, Shen J, Hua T, Zhang T, Yang X. 3D Reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl Catal B-Environ. 2018;232:562.
Yang X, Tian L, Zhao X, Tang H, Liu Q, Li G. Interfacial optimization of g-C3N4-based Z-scheme heterojunction toward synergistic enhancement of solar-driven photocatalytic oxygen evolution. Appl Catal B-Environ. 2019;244:240.
Svoboda J. Magnetic Techniques for the Treatment of Materials. Berlin: Springer; 2004. 1.
Svoboda J, Fujita T. Recent developments in magnetic methods of material separation. Miner Eng. 2003;16(9):785.
Hillier S, Hodson ME. High-gradient magnetic separation applied to sand-size particles; an example of feldspar separation from mafic minerals. J Sediment Res. 1997;67(5):975.
Kolm H, Oberteuffer J, Kelland D. High-gradient magnetic separation. Sci Am. 1975;233(5):46.
Fletcher D. Fine particle high gradient magnetic entrapment. IEEE Trans Magn. 1991;27(4):3655.
Hubbuch JJ, Matthiesen DB, Hobley TJ, Thomas OR. High gradient magnetic separation versus expanded bed adsorption: a first principle comparison. Bioseparation. 2001;10(1):99.
Moeser GD, Roach KA, Green WH, Alan Hatton T, Laibinis PE. High-gradient magnetic separation of coated magnetic nanoparticles. AIChE J. 2004;50(11):2835.
Majewski P, Thierry B. Functionalized magnetite nanoparticles—synthesis, properties, and bio-applications. Crit Rev Solid State. 2007;32(3–4):203.
Ngomsik AF, Bee A, Draye M, Cote G, Cabuil V. Magnetic nano- and microparticles for metal removal and environmental applications: a review. Comptes Rendus Chim. 2005;8(6):963.
Menini L, Pereira MC, Parreira LA, Fabris JD, Gusevskaya EV. Cobalt- and manganese-substituted ferrites as efficient single-site heterogeneous catalysts for aerobic oxidation of monoterpenic alkenes under solvent-free conditions. J Catal. 2008;254(2):355.
Stein M, Wieland J, Steurer P, Tölle F, Mülhaupt R, Breit B. Iron nanoparticles supported on chemically-derived graphene: catalytic hydrogenation with magnetic catalyst separation. Adv Synth Catal. 2011;353(4):523.
Rossi LM, Costa NJS, Silva FP, Wojcieszak R. Magnetic nanomaterials in catalysis: advanced catalysts for magnetic separation and beyond. Green Chem. 2014;16(6):2906.
Safarik I, Safarikova M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn Res Technol. 2004;2(1):7.
Hajian R, Ehsanikhah A. Manganese porphyrin immobilized on magnetic MCM-41 nanoparticles as an efficient and reusable catalyst for alkene oxidations with sodium periodate. Chem Phys Lett. 2018;691:146.
Ma Q, Cui Y, Deng X, Li B, Cheng Q, Cheng X. Fabrication of magnetic TiO2 nano-catalyst and its enhanced photocatalytic and recycle performance. J Nanosci Nanotechnol. 2017;17(3):2019.
Chang YC, Chen DH. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J Colloid Interface Sci. 2005;283(2):446.
Liu JF, Zhao ZS, Jiang GB. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ Sci Technol. 2008;42(18):6949.
Hudlet S, Jean MS, Roulet B, Berger J, Guthmann C. Electrostatic forces between metallic tip and semiconductor surfaces. J Appl Phys. 1995;77(7):3308.
Martín A, Martínez F, Malfeito J, Palacio L, Prádanos P, Hernández A. Zeta potential of membranes as a function of pH: optimization of isoelectric point evaluation. J Membr Sci. 2003;213(1):225.
Igor C, Laurence DE, Lazare NO, Jean-François F, Simone CJ, Martin S, Hervé M, Pierre D. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst. 2005;130(10):1395.
Zeng W, Feng LP, Su J, Pan HX, Liu ZT. Layer-controlled and atomically thin WS2 films prepared by sulfurization of atomic-layer-deposited WO3 films. J Alloy Compd. 2018;745:834.
Rezaee M, Khoie SMM, Liu KH. The role of brookite in mechanical activation of anatase-to-rutile transformation of nanocrystalline TiO2: an XRD and Raman spectroscopy investigation. CrystEngComm. 2011;13(16):5055.
Xu S, Sun J, Weng L, Hua Y, Liu W, Neville A, Hu M, Gao X. In-situ friction and wear responses of WS2 films to space environment: vacuum and atomic oxygen. Appl Surf Sci. 2018;447:368.
Yen PC, Huang YS, Tiong KK. The growth and characterization of rhenium-doped WS2 single crystals. J Phys: Condens Matter. 2004;16(12):2171.
Yang L, Majumdar K, Liu H, Du Y, Wu H, Hatzistergos M, Hung PY, Tieckelmann R, Tsai W, Hobbs C. Chloride molecular doping technique on 2D materials: WS2 and MoS2. Nano Lett. 2014;14(11):6175.
Karikalan N, Karthik R, Chen SM, Karuppiah C, Elangovan A. Sonochemical synthesis of sulfur doped reduced graphene oxide supported CuS nanoparticles for the non-enzymatic glucose sensor applications. Sci Rep. 2017;7(1):2494.
Wang Q, Ning A, Yan B, Hang H, Li J, Lu X, Liu Y, Wang F, Li Z, Lei Z. High photocatalytic hydrogen production from methanol aqueous solution using the photocatalysts CuS/TiO2. Int J Hydrog Energy. 2013;38(25):10739.
Wen Z, Shao P, Ci S, Yi L, Cai P, Huang P, Cao C. Hollow CuS microcube electrocatalysts for CO2 reduction reaction. Chemelectrochem. 2017;4(10):2593.
Zhang S, Hongyu LI, Qin Z. Promotional effect of F-doped V2O5–WO3/TiO2 catalyst for NH3-SCR of NO at low-temperature. Appl Catal A-Gen. 2012;435(17):156.
Wang J, Chen Y, Zhou W, Tian G, Xiao Y, Fu H, Fu H. Cubic quantum dot/hexagonal microsphere ZnIn2S4 heterophase junction for exceptional visible-light-driven photocatalytic H2 evolution. J Mater Chem A. 2017;5(18):8451.
Zhang Q, Hu S, Fan Z, Liu D, Zhao Y, Ma H, Li F. Preparation of g-C3N4/ZnMoCdS hybrid heterojunction catalyst with outstanding nitrogen photofixation performance under visible light via hydrothermal post-treatment. Dalton Trans. 2016;45(8):3497.
Qi D, Lu L, Xi Z, Wang L, Zhang J. Enhanced photocatalytic performance of TiO2 based on synergistic effect of Ti3+ self-doping and slow light effect. Appl Catal B-Environ. 2014;160(6):621.
Li K, Gao S, Wang Q, Xu H, Wang Z, Huang B, Dai Y, Lu J. In-situ-reduced synthesis of Ti3+ self-doped TiO2/g-C3N4 heterojunctions with high photocatalytic performance under LED light irradiation. ACS Appl Mater Interfaces. 2015;7(17):9023.
An L, Li Y, Luo M, Yin J, Zhao YQ, Xu C, Cheng F, Yang Y, Xi P, Guo S. Atomic-level coupled interfaces and lattice distortion on CuS/NiS2 nanocrystals boost oxygen catalysis for flexible Zn-air batteries. Adv Funct Mater. 2017;27(42):1703779.
Xing M, Xu W, Dong C, Bai Y, Zeng J, Zhou Y, Zhang J, Yin Y. Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem. 2018;4(6):1359.
Acknowledgements
This work was financially supported by the State Key Research Development Program of China (No. 2016YFA0204200), the National Natural Science Foundation of China (Nos. 21822603, 21773062, 21577036, 21377038 and 21237003), Shanghai Pujiang Program (No. 17PJD011) and the Fundamental Research Funds for the Central Universities (No. 22A201514021).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ji, JH., Xiao, YF., Shen, B. et al. Magnetic separation of metal sulfides/oxides by Fe3O4 at room temperature and atmospheric pressure. Rare Met. 38, 379–389 (2019). https://doi.org/10.1007/s12598-019-01232-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-019-01232-3