Abstract
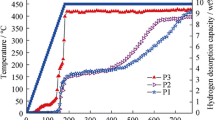
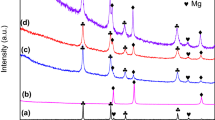
In order to enhance the hydrogen storage properties of LiBH4, activated charcoal (AC) was used as the scaffold to confine LiBH4 in this paper. Ball milling was used to prepare LiBH4/AC composites. Experimental results show that dehydrogenation properties of ball-milled LiBH4/AC (LiBH4/AC-BM) are greatly improved compared with that of pristine LiBH4, ball-milled LiBH4 (LiBH4-BM) and hand-milled LiBH4/AC (LiBH4/AC-HM). The onset dehydrogenation temperature of LiBH4 for LiBH4/AC-BM is around 160 °C, which is 170 °C lower than that of pristine LiBH4. At around 400 °C, LiBH4/AC-BM finishes the dehydrogenation with a hydrogen capacity of 13.6 wt%, which is approximately the theoretical dehydrogenation capacity of pure LiBH4 (13.8 wt%), while the dehydrogenation processes for LiBH4-BM and LiBH4/AC-BM do not finish even when they were heated to 600 °C. The isothermal dehydriding measurements show that it takes only 15 min for LiBH4/AC-BM to reach a dehydrogenation capacity of 10.1 wt% at 350 °C, whereas the pristine LiBH4 and the LiBH4/AC-HM release hydrogen less than 1 wt% under the same conditions. The dehydrogenation process and the effect of AC were discussed.






Similar content being viewed by others
References
Schlapbach L, Zuttel A. Hydrogen-storage materials for mobile applications. Nature. 2001;414(6861):353.
Orimo S, Nakamori Y, Eliseo JR, Züttel A, Jensen CM. Complex hydrides for hydrogen storage. Chem Rev. 2007;107(10):4111.
Yang J, Sudik A, Wolverton C, Siegel DJ. High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Chem Soc Rev. 2010;39(2):656.
Seayad AM, Antonelli DM. Recent advances in hydrogen storage in metal-containing inorganic nanostructures and related materials. Adv Mater. 2004;16(9–10):765.
Durbin DJ, Malardier-Jugroot C. Review of hydrogen storage techniques for on board vehicle applications. Int J Hydrog Energy. 2013;38(34):14595.
Crabtree GW, Dresselhaus MS, Buchanan MV. The hydrogen economy. Phys Today. 2004;57(12):39.
Jena P. Materials for hydrogen storage: past, present, and future. J Phys Chem Lett. 2011;2(3):206.
Vajo JJ, Olson GL. Hydrogen storage in destabilized chemical systems. Scripta Mater. 2007;56(10):829.
Cui J, Wang H, Sun DL, Zhang QA, Zhu M. Realizing nano-confinement of magnesium for hydrogen storage using vapour transport deposition. Rare Met. 2016;35(5):401.
Jiang WQ, Guo J, Zhou ZC, Wei YY. Hydrogen storage properties of as-cast and annealed low-Co La1.8Ti0.2MgNi8.9Co0.1 alloys. Rare Met. 2016;35(7):559.
Li YM, Zhang HW, Zhang YH, Liu ZC, Ren HP. Changes of crystal structure and hydrogen storage performances for multiphase La0.7Mg0.3Ni3 alloy upon gas-solid cycling. Rare Met. 2017;36(2):101.
Zhu XL, Han SM, Zhao X, Li Y, Liu BZ. Improving hydrogen storage performance of Li–Mg–N–H system by adding niobium hydride. Rare Met. 2014;33(1):86.
Sun J, Xiao XZ, Zheng ZJ, Fan XL, Xu CC, Liu LX, Li SQ, Chen LX. Synthesis of nanoscale CeAl4 and its high catalytic efficiency for hydrogen storage of sodium alanate. Rare Met. 2017;36(2):77.
Ouyang LZ, Xu YJ, Dong HW, Sun LX, Zhu M. Production of hydrogen via hydrolysis of hydrides in Mg–La system. Int J Hydrog Energy. 2009;34(24):9671.
Ouyang LZ, Wen YJ, Xu YJ, Yang XS, Sun LX, Zhu M. The effect of Ni and Al addition on hydrogen generation of Mg3La hydrides via hydrolysis. Int J Hydrog Energy. 2010;35(15):8161.
Zhong H, Wang H, Liu JW, Sun DL, Fang F, Zhang QA, Ouyang LZ, Zhu M. Enhanced hydrolysis properties and energy efficiency of MgH2-base hydrides. J Alloys Compd. 2016;680:419.
Huang MH, Ouyang LZ, Wang H, Liu JW, Zhu M. Hydrogen generation by hydrolysis of MgH2 and enhanced kinetics performance of ammonium chloride introducing. Int J Hydrog Energy. 2015;40(18):6145.
Ouyang LZ, Huang JM, Wang H, Wen YJ, Zhang QA, Sun DL, Zhu M. Excellent hydrolysis performances of Mg3RE hydrides. Int J Hydrog Energy. 2013;38(7):2973.
Ouyang LZ, Zhong H, Li ZM, Cao ZJ, Wang H, Liu JW, Zhu XK, Zhu M. Low-cost method for sodium borohydride regeneration and the energy efficiency of its hydrolysis and regeneration process. J Power Sources. 2014;269:768.
Huang JM, Duan RM, Ouyang LZ, Wen YJ, Wang H, Zhu M. The effect of particle size on hydrolysis properties of Mg3La hydrides. Int J Hydrog Energy. 2014;39(25):13564.
Huang JM, Ouyang LZ, Wen YJ, Wang H, Liu JW, Chen ZL, Zhu M. Improved hydrolysis properties of Mg3RE hydrides alloyed with Ni. Int J Hydrog Energy. 2014;39(13):6813.
Li HW, Yan YG, Orimo S, Züttel A, Jensen CM. Recent progress in metal borohydrides for hydrogen storage. Energies. 2011;4(12):185.
Züttel A, Borgschulte A, Orimo S. Tetrahydroborates as new hydrogen storage materials. Scripta Mater. 2007;56(10):823.
Graetz J. New approaches to hydrogen storage. Chem Soc Rev. 2009;38(1):73.
Rönnebro E. Development of group II borohydrides as hydrogen storage materials. Curr Opin Solid State Mater Sci. 2011;15(2):44.
Rude LH, Nielsen TK, Ravnsbaek DB, Bösenberg U, Ley MB, Richter B, Arnbjerg LM, Dornheim M, Filinchuk Y, Besenbacher F, Jensen TR. Tailoring properties of borohydrides for hydrogen storage: a review. Physica Status Solidi (a). 2011;208(8):1754.
Jain IP, Jain P, Jain A. Novel hydrogen storage materials: a review of lightweight complex hydrides. J Alloys Compd. 2010;503(2):303.
Vajo JJ, Skeith SL, Mertens F. Reversible storage of hydrogen in destabilized LiBH4. J Phys Chem B. 2005;109(9):3719.
Alapati SV, Karl Johnson J, Sholl DS. Using first principles calculations to identify new destabilized metal hydride reactions for reversible hydrogen storage. Phys Chem Chem Phys. 2007;9(12):1438.
Li HW, Kikuchi K, Nakamori Y, Ohba N, Miwa K, Towata S, Orimo S. Dehydriding and rehydriding processes of well-crystallized Mg(BH4)2 accompanying with formation of intermediate compounds. Acta Mater. 2008;56(6):1342.
Alapati SV, Johnson JK, Sholl DS. Large-Scale screening of metal hydride mixtures for high-capacity hydrogen storage from first-principles calculations. J Phys Chem C. 2008;112(14):5258.
Gennari FC. Destabilization of LiBH4 by MH2 (M = Ce, La) for hydrogen storage: nanostructural effects on the hydrogen sorption kinetics. Int J Hydrog Energy. 2011;36(23):15231.
Zhou YF, Liu YF, Wu W, Zhang Y, Gao MX, Pan HG. Improved hydrogen storage properties of LiBH4 destabilized by in situ formation of MgH2 and LaH3. J Phys Chem C. 2011;116(1):1588.
Li L, Zhang ZC, Wang YJ, Jiao LF, Yuan HT. Direct synthesis and dehydrogenation properties of NaAlH4 catalyzed with ball-milled Ti-B. Rare Met. 2017;36(6):517.
Zhou H, Liu HZ, Xu L, Gao SC, Wang XH, Yan M. Hydrogen storage properties of Nb-compounds-catalyzed LiBH4–MgH2. Rare Met. 2017;36(9):723.
Nielsen TK, Bösenberg U, Gosalawit R, Dornheim M, Cerenius Y, Besenbacher F, Jensen TR. A reversible nanoconfined chemical reaction. ACS Nano. 2010;4(7):3903.
Nielsen TK, Javadian P, Polanski M, Besenbacher F, Bystrzycki J, Jensen TR. Nanoconfined NaAlH4: determination of distinct prolific effects from pore size, crystallite size, and surface interactions. J Phys Chem C. 2012;116(39):21046.
Shane DT, Corey RL, McIntosh C, Rayhel LH, Bowman RC, Vajo JJ, Gross AF, Conradi MS. LiBH4 in carbon aerogel nanoscaffolds: an NMR study of atomic motions. J Phys Chem C. 2010;114(9):4008.
Ngene P, Adelhelm P, Beale AM, de Jong KP, De Jongh EP. LiBH4/SBA-15 nanocomposites prepared by melt infiltration under hydrogen pressure: synthesis and hydrogen sorption properties. J Phys Chem C. 2010;114(13):6163.
Bardají EG, Zhao-Karger Z, Boucharat N, Nale A, Van Setten MJ, Lohstroh W, Röhm E, Catti M, Fichtner M. LiBH4–Mg(BH4)2: a physical mixture of metal borohydrides as hydrogen storage material. J Phys Chem C. 2011;115(13):6095.
Brinks HW, Fossdal A, Hauback BC. Adjustment of the stability of complex hydrides by anion substitution. J Phys Chem C. 2008;112(14):5658.
Van Setten MJ, de Wijs GA, Brocks G. Ab initio study of the effects of transition metal doping of Mg2NiH4. Phys Rev B. 2007;76(7):8.
Yu XB, Wu Z, Chen QR, Li ZL, Weng BC, Huang TS. Improved hydrogen storage properties of LiBH4 destabilized by carbon. Appl Phys Lett. 2007;90(3):034106.
Zhang Y, Zhang W, Wang A, Sun LX, Fan M, Chu H, Sun J, Zhang T. LiBH4 nanoparticles supported by disordered mesoporous carbon: hydrogen storage performances and destabilization mechanisms. Int J Hydrog Energy. 2007;32(16):3976.
Agresti F, Khandelwal A, Capurso G, Russo SL, Maddalena A, Principi G. Improvement of dehydrogenation kinetics of LiBH(4) dispersed on modified multi-walled carbon nanotubes. Nanotechnology. 2010;21(6):065707.
Liu X, Peaslee D, Jost CZ, Majzoub EH. Controlling the decomposition pathway of LiBH4 via confinement in highly ordered nanoporous carbon. J Phys Chem C. 2010;114(33):14036.
Cento C, Gislon P, Bilgili M, Masci A, Zheng Q, Prosini PP. How carbon affects hydrogen desorption in NaAlH4 and Ti-doped NaAlH4. J Alloys Compd. 2007;437(1–2):360.
Pukazhselvan D, Gupta BK, Srivastava A, Srivastava ON. Investigations on hydrogen storage behavior of CNT doped NaAlH4. J Alloys Compd. 2005;403(1–2):312.
Xu J, Meng R, Cao J, Gu X, Qi Z, Wang W, Chen Z. Enhanced dehydrogenation and rehydrogenation properties of LiBH4 catalyzed by graphene. Int J Hydrog Energy. 2013;38(6):2796.
Pierard N, Fonseca A, Colomer JF, Bossuot C, Benoit JM, Van Tendeloo G, Pirard JP, Nagy JB. Ball milling effect on the structure of single-wall carbon nanotubes. Carbon. 2004;42(8–9):1691.
Tao Z, Geng H, Yu K, Yang Z, Wang Y. Effects of high-energy ball milling on the morphology and the field emission property of multi-walled carbon nanotubes. Mater Lett. 2004;58(27–28):3410.
Balema VP, Pecharsky AO, Pecharsky VK. Concerning the transformations of Ti3Ir alloy during high-energy ball-milling. J Alloys Compd. 2000;307(1–2):184.
Züttel A, Rentsch S, Fischer P, Wenger P, Sudan P, Mauron P, Emmenegger C. Hydrogen storage properties of LiBH4. J Alloys Compd. 2003;356–357:515.
Shao J, Xiao X, Fan X, Zhang L, Li S, Ge H, Wang Q, Chen L. Low-temperature reversible hydrogen storage properties of LiBH4: a synergetic effect of nanoconfinement and nanocatalysis. J Phys Chem C. 2014;118(21):11252.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 51471149 and 51171168) and the Public Project of Zhejiang Province (No. 2015C31029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, H., Wang, XH., Liu, HZ. et al. Improved hydrogen storage properties of LiBH4 confined with activated charcoal by ball milling. Rare Met. 38, 321–326 (2019). https://doi.org/10.1007/s12598-018-1067-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-018-1067-1