Abstract
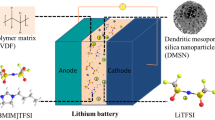
In order to avoid leakage problem caused by liquid electrolyte, a new ionogel electrolyte was developed by in situ immobilizing organosilicon-functionalized ionic liquid within a nanoporous silica matrix. The ionic liquid evenly coats on the surface of porous silica and fills in the silica framework pores with no strong chemical interaction. The ionogel electrolyte has the dual advantages of a silica solid support and a wide electrochemical stability window of ionic liquid (4.87 V vs. Li+/Li). The half-cells assembled with this electrolyte and LiFePO4 electrode have excellent performance at room temperature and 60 °C. The Li/SiO2-IGE/LiFePO4 cell displays a discharge capacity of 129.1 mAh·g−1 after 200 charge/discharge cycles at room temperature.






Similar content being viewed by others
References
Gu J, Li B, Du Z, Zhang C, Zhang D, Yang S. Multi-atomic layers of metallic aluminum for ultralong life lithium storage with high volumetric capacity. Adv Funct Mater. 2017;27(27):1700840.
Gu J, Du Z, Zhang C, Yang S. Pyridinic nitrogen-enriched carbon nanogears with thin teeth for superior lithium storage. Adv Energy Mater. 2016;6(18):1600917.
Li B, Zhang G, Huang K, Qiao L, Pang H. One-step synthesis of CoSn(OH)6 nanocubes for high-performance all solid-state flexible supercapacitors. Rare Met. 2017;36(5):457.
Sun J, Yang S, Li S, Cao B. Double-activated porous carbons for high-performance supercapacitor electrodes. Rare Met. 2017;36(5):449.
Zhang L, Wu S, Wan Y, Huo Y, Luo Y, Yang M, Li M, Lu Z. Mn3O4/carbon nanotube nanocomposites recycled from waste alkaline Zn–MnO2 batteries as high-performance energy materials. Rare Met. 2017;36(5):442.
Wu F, Zhu Q, Chen R, Chen N, Chen Y, Li L. Ionic liquid electrolytes with protective lithium difluoro(oxalate)borate for high voltage lithium-ion batteries. Nano Energy. 2015;13:546.
Eftekhari A, Liu Y, Chen P. Different roles of ionic liquids in lithium batteries. J Power Sour. 2016;334:221.
Chavan SN, Tiwari A, Nagaiah TC, Mandal D. Ether and siloxane functionalized ionic liquids and their mixtures as electrolyte for lithium-ion batteries. Phys Chem Chem Phys. 2016;18(24):16116.
Giffin GA. Ionic liquid-based electrolytes for “beyond lithium” battery technologies. J Mater Chem A. 2016;4(35):13378.
Wang Y, Zhong W-H. Development of electrolytes towards achieving safe and high-performance energy-storage devices: a review. ChemElectroChem. 2015;2(1):22.
Chen R, Qu W, Qian J, Chen N, Dai Y, Guo C, Huang Y, Li L, Wu F. Zirconia-supported solid-state electrolytes for high-safety lithium secondary batteries in a wide temperature range. J Mater Chem A. 2017;5(47):24677.
Wu F, Tan G, Chen R, Li L, Xiang J, Zheng Y. Novel solid-state Li/LiFePO(4) battery configuration with a ternary nanocomposite electrolyte for practical applications. Adv Mater. 2011;23(43):5081.
Wu F, Chen N, Chen R, Zhu Q, Tan G, Li L. Self-regulative nanogelator solid electrolyte: a new option to improve the safety of lithium battery. Adv Sci (Weinh). 2016;3(1):1500306.
Khodagholy D, Curto VF, Fraser KJ, Gurfinkel M, Byrne R, Diamond D, Malliaras GG, Benito-Lopez F, Owens RM. Organic electrochemical transistor incorporating an ionogel as a solid state electrolyte for lactate sensing. J Mater Chem. 2012;22(10):4440.
Wang S, Hsia B, Carraro C, Maboudian R. High-performance all solid-state micro-supercapacitor based on patterned photoresist-derived porous carbon electrodes and an ionogel electrolyte. J Mater Chem A. 2014;2(21):7997.
Le Bideau J, Ducros JB, Soudan P, Guyomard D. Solid-state electrode materials with ionic-liquid properties for energy storage: the lithium solid-state ionic-liquid concept. Adv Funct Mater. 2011;21(21):4073.
Kumar A, Ramani VK. RuO2–SiO2 mixed oxides as corrosion-resistant catalyst supports for polymer electrolyte fuel cells. Appl Catal B. 2013;138–139:43.
Zheng B, Li W, Liu L, Wang X, Chen C, Yu Z, Li H. Determination of phenols isomers in water by novel nanosilica/polydimethylsiloxane-coated stirring bar combined with high performance liquid chromatography-fourier transform infrared spectroscopy. Sci Reports. 2017;7(1):8697.
Miao Z, Shi J, Hao J, Fan Q, Zhang Y, Zhang M. Effect of nano-TiO2 and nano-SiO2 addition on the morphological control of α-Al2O3 platelets via solid-state reaction. Ceram Int. 2016;42(1):1183.
Rong M, Zhang M, Zheng Y, Zeng H, Friedrichc K. Improvement of tensile properties of nano-SiO2/PP composites in relation to percolation mechanism. Polym Commun. 2001;42(7):3301.
Chattopadhyay DK, Webster DC. Thermal stability and flame retardancy of polyurethanes. Prog Polym Sci. 2009;34(10):1068.
Di Noto V, Lavina S, Giffin GA, Negro E, Scrosati B. Polymer electrolytes: present, past and future. Electrochim Acta. 2011;57:4.
Afify ND, Dalba G, Mahendra Kumar Koppolu U, Armellini C, Jestin Y, Rocca F. XRD and EXAFS studies of crystallisation in films. And EXAFS studies of crystallisation in films. Mater Sci Semicond Process. 2006;9(6):1043.
Chen X, Zhao J, Zhang J, Qiu L, Xu D, Zhang H, Han X, Sun B, Fu G, Zhang Y, Yan F. Bis-imidazolium based poly(ionic liquid) electrolytes for quasi-solid-state dye-sensitized solar cells. J Mater Chem. 2012;22(34):18018.
Leones R, Costa CM, Machado AV, Esperança JMSS, Silva MM, Lanceros-Méndez S. Development of solid polymer electrolytes based on poly(vinylidene fluoride-trifluoroethylene) and the [N1 1 1 2(OH)][NTf2] ionic liquid for energy storage applications. Solid State Ionics. 2013;253:143.
Li Z, Xia Q, Liu L, Lei G, Xiao Q, Gao D, Zhou X. Effect of zwitterionic salt on the electrochemical properties of a solid polymer electrolyte with high temperature stability for lithium ion batteries. Electrochim Acta. 2010;56(2):804.
Yong T, Zhang L, Wang J, Mai Y, Yan X, Zhao X. Novel choline-based ionic liquids as safe electrolytes for high-voltage lithium-ion batteries. J Power Sour. 2016;328:397.
Zhang P, Yang LC, Li LL, Ding ML, Wu YP, Holze R. Enhanced electrochemical and mechanical properties of P(VDF-HFP)-based composite polymer electrolytes with SiO2 nanowires. J Membr Sci. 2011;379(1–2):80.
Ma P, Tan J, Cheng H, Fang Y, Wang Y, Dai Y, Fang S, Zhou X, Lin Y. Polyaniline-grafted silica nanocomposites-based gel electrolytes for quasi-solid-state dye-sensitized solar cells. Appl Surf Sci. 2018;427:458.
Wu PW, Holm SR, Duong AT, Dunn B, Kaner RB. ChemInform Abstract: A sol-gel solid electrolyte with high lithium ion conductivity. Cheminform. 1997;28(27):1004.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2016YFB0100204), the National Natural Science Foundation of China (No. 51772030), the Joint Funds of the National Natural Science Foundation of China (No. U1564206), the Major Achievements Transformation Project for Central University in Beijing and the Science and Technology Project of State Grid Corporation of China (No. 15-JS-191).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, YJ., Guo, C., Yue, LS. et al. Organosilicon-group-derived silica-ionogel electrolyte for lithium ion batteries. Rare Met. 37, 504–509 (2018). https://doi.org/10.1007/s12598-018-1056-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-018-1056-4