Abstract
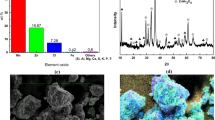
Alkaline zinc manganese dioxide (Zn–MnO2) batteries are widely used in everyday life. Recycling of waste alkaline Zn–MnO2 batteries has always been a hot environmental concern. In this study, a simple and cost-effective process for synthesizing Mn3O4/carbon nanotube (CNT) nanocomposites from recycled alkaline Zn–MnO2 batteries is presented. Manganese oxide was recovered from spent Zn–MnO2 battery cathodes. The Mn3O4/CNT nanocomposites were produced by ball milling the recovered manganese oxide in a commercial multi-wall carbon nanotubes (MWCNTs) solution. Scanning electron microscopy (SEM) analysis demonstrates that the nanocomposite has a unique three-dimensional (3D) bird nest structure. Mn3O4 nanoparticles are homogeneously distributed on MWCNT framework. Mn3O4/CNT nanocomposites were evaluated as an anode material for lithium-ion batteries, exhibiting a highly reversible specific capacitance of ~580 mAh·g−1 after 100 cycles. Moreover, Mn3O4/CNT nanocomposite also shows a fairly positive onset potential of −0.15 V and quite high oxygen reducibility when considered as an electrocatalyst for oxygen reduction reaction.





Similar content being viewed by others
References
Biswas RK, Karmakar AK, Kumar SL. Recovery of manganese and zinc from spent Zn–C cell powder: experimental design of leaching by sulfuric acid solution containing glucose. Waste Manag. 2016;51:174.
Sun MX, Wang YT, Hong JL, Dai JL, Wang RQ, Niu ZR, Xin BP. Life cycle assessment of a bio-hydrometallurgical treatment of spent Zn–Mn batteries. J Clean Prod. 2016;129:350.
Sobianowska-Turek A, Szczepaniak W, Sobianowska K, Maciejewski P. Recovery of K, Na, Mn and Zn from spent batteries by leaching with water. Przem Chem. 2015;94(5):702.
Xin BP, Jiang WF, Aslam H, Zhang K, Liu CH, Wang RQ, Wang YT. Bioleaching of zinc and manganese from spent Zn–Mn batteries and mechanism exploration. Bioresour Technol. 2012;106:147.
Sobianowska-Turek A, Szczepaniak W, Marcinkowski TA, Zablocka-Malicka M. Recovery of Mn and Zn by reductive acid leaching of spent batteries. Przem Chem. 2013;92(2):248.
Falco L, Quina MJ, Gando-Ferreira LM, Thomas H, Curutchet G. Solvent extraction studies for separation of Zn(II) and Mn(II) from spent batteries leach solutions. Sep Sci Technol. 2014;49(3):398.
Formanek J, Jandova J, Sis J. A review of hydrometallurgical technologies for the recovery of Zn and Mn from spent alkaline and zinc batteries. Chem Listy. 2012;106(5):350.
Cao X, Guo GH, Liu FF, Zhou Y, Zhang SS. The properties of LiMn2O4 synthesized by molten salt method using MnO2 as manganese source recycled from spent Zn–Mn batteries. Int J Electrochem Sci. 2015;10(5):3841.
Qu J, Feng Y, Zhang Q, Cong Q, Luo CQ, Yuan X. A new insight of recycling of spent Zn–Mn alkaline batteries: synthesis of Zn x Mn1−x O nanoparticles and solar light driven photocatalytic degradation of bisphenol A using them. J Alloys Compd. 2015;622:703.
Song YN, Huang QF, Niu ZR, Ma J, Xin BP, Chen S, Dai JL, Wang RQ. Preparation of Zn–Mn ferrite from spent Zn–Mn batteries using a novel multi-step process of bioleaching and co-precipitation and boiling reflux. Hydrometallurgy. 2015;153:66.
Xi GX, Xi YB, Xu HD, Wang L. Study of the preparation of Ni–Mn–Zn ferrite using spent Ni–MH and alkaline Zn–Mn batteries. J Magn Magn Mater. 2016;398:196.
Gabal MA, Al-Luhaibi RS, Al Angari YM. Effect of Zn-substitution on the structural and magnetic properties of Mn–Zn ferrites synthesized from spent Zn–C batteries. J Magn Magn Mater. 2013;348:107.
Hu P, Pan DA, Zhang SG, Tian JJ, Volinsky AA. Mn–Zn soft magnetic ferrite nanoparticles synthesized from spent alkaline Zn–Mn batteries. J Alloys Compd. 2011;509(9):3991.
Kim TH, Kang JG, Sohn JS, Rhee KI, Lee SW, Shin SM. Preparation of Mn–Zn ferrite from spent zinc–carbon batteries by alkali leaching, acid leaching and co-precipitation. Met Mater Int. 2008;14(5):655.
Nan JM, Han DM, Cui M, Yang MJ, Pan LM. Recycling spent zinc manganese dioxide batteries through synthesizing Zn–Mn ferrite magnetic materials. J Hazard Mater. 2006;133(1–3):257.
Chen S, Guo GH, Liu FF. Study on the performance of LiCo x Mn2−x O4−y F y using spent alkaline Zn–Mn batteries as manganese source. Solid State Ionics. 2014;261:59.
Deep A, Sharma AL, Mohanta GC, Kumar P, Kim KH. A facile chemical route for recovery of high quality zinc oxide nanoparticles from spent alkaline batteries. Waste Manag. 2016;51:190.
Yang M, Zhang GL, Li PF, Li XY, Chen C. Synthesis of spherical assembly composed of MnO2 nanoparticles using spent Zn–Mn batteries. Appl Mech Mater. 2012;178–181:1012.
Gabal MA, Al-luhaibi RS, Al Angari YM. Mn–Zn nano-crystalline ferrites synthesized from spent Zn–C batteries using novel gelatin method. J Hazard Mater. 2013;246:227.
Ma Y, Cui Y, Zuo X, Huang S, Hu K, Xiao X, Nan J. Reclaiming the spent alkaline zinc manganese dioxide batteries collected from the manufacturers to prepare valuable electrolytic zinc and LiNi0.5Mn1.5O4 materials. Waste Manag. 2014;34(10):1793.
Vincent C, Scrosati B. Modern Batteries—An Introduction to Electrochemical Power Sources. Arnold: Elsevier; 1997. 1.
Wang HL, Cui LF, Yang YA, Casalongue HS, Robinson JT, Liang YY, Cui Y, Dai HJ. Mn3O4-graphene hybrid as a high-capacity anode material for lithium ion batteries. J Am Chem Soc. 2010;132(40):13978.
Wang YJ. Coprecipitated 3D nanostructured graphene oxide-Mn3O4 hybrid as anode of lithium-ion batteries. J Mater Res. 2015;30(4):484.
Nagamuthu S, Vijayakumar S, Muralidharan G. Synthesis of Mn3O4/amorphous carbon nanoparticles as electrode material for high performance supercapacitor applications. Energy Fuel. 2013;27(6):3508.
Lee JW, Hall AS, Kim JD, Mallouk TE. A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem Mater. 2012;24(6):1158.
Gorlin Y, Chung CJ, Nordlund D, Clemens BM, Jaramillo TF. Mn3O4 supported on glassy carbon: an active non-precious metal catalyst for the oxygen reduction reaction. ACS Catal. 2012;2(12):2687.
Morelos-Gomez A, Fujishige M, Vega-Diaz SM, Ito I, Fukuyo T, Cruz-Silva R, Tristan-Lopez F, Fujisawa K, Fujimori T, Futamura R, Kaneko K, Takeuchi K, Hayashi T, Kim YA, Terrones M, Endo M, Dresselhaus MS. High electrical conductivity of double-walled carbon nanotube fibers by hydrogen peroxide treatments. J Mater Chem A. 2016;4(1):74.
Sato-Berru RY, Vazquez-Olmos A, Fernandez-Osorio AL, Sotres-Martinez S. Micro-Raman investigation of transition-metal-doped ZnO nanoparticles. J Raman Spectrosc. 2007;38(9):1073.
Liang X, Wen ZY, Liu Y, Wu MF, Jin J, Zhang H, Wu XW. Improved cycling performances of lithium sulfur batteries with LiNO3-modified electrolyte. J Power Sour. 2011;196(22):9839.
Lima FHB, Calegaro ML, Ticianelli EA. Investigations of the catalytic properties of manganese oxides for the oxygen reduction reaction in alkaline media. J Electroanal Chem. 2006;590(2):152.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21671096 and 21603094), the Shenzhen Peacock Plan (No. KQCX20140522150815065), the Natural Science Foundation of Shenzhen (Nos. JCYJ20150630145302231 and JCYJ20150331101823677) and the Science and Technology Innovation Foundation for the Undergraduates of South University of Science and Technology of China (Nos. 2016S10, 2016S20, 2015x19 and 2015x12).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, LH., Wu, SS., Wan, Y. et al. Mn3O4/carbon nanotube nanocomposites recycled from waste alkaline Zn–MnO2 batteries as high-performance energy materials. Rare Met. 36, 442–448 (2017). https://doi.org/10.1007/s12598-017-0902-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-017-0902-0