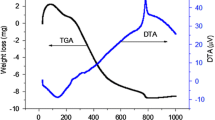
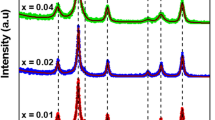
Abstract
LaFe1−x−y Co x Pd y O3 [(x, y) = (0, 0), (0.40, 0), (0.38, 0.05)] nanoparticles were synthesized via a co-precipitation route using ammonium hydroxide, sodium hydroxide and ammonium carbonate as the precipitant and calcination at different temperatures to study the compositional driven structural changes in lanthanum ferrites. Analysis of X-ray diffraction (XRD) patterns confirms the formation of single-phase perovskite structure and existence of orthorhombic Pnma symmetry for calcined powders. Field emission scanning electron microscope (FESEM) observations show that Pd-doped powders yield finer particles along with narrower particle size distribution compared with LaFeO3 and LaFe0.6Co0.4O3. Moreover, using ammonia as the precipitant leads to a smaller mean particle size of powders compared to NaOH, as well as significant difference in morphology of the particles. Raman analysis reveals that both Co and Pd atoms substitute Fe site in perovskite structure with shifting of phonon modes. Comparing Raman spectra demonstrates the presence of more oxygen vacancies in Pd-doped perovskites. It can be concluded from the results that Pd is successfully incorporated into the perovskite structure by co-precipitation method.









Similar content being viewed by others
References
Royer S, Duprez D, Can F, Courtois X, Batiot-Dupeyrat C, Laassiri S, Alamdari H. Perovskites as substitutes of noble metals for heterogeneous catalysis: dream or reality. Chem Rev. 2014;114(20):10292.
Zheng Y, Ran R, Shao Z. Cr doping effect in B-site of La0.75Sr0.25MnO3 on its phase stability and performance as an SOFC anode. Rare Metals. 2009;28(4):361.
Sun H-Y, Sen W, Ma W-H, Yu J, Yang J-J. Fabrication of LSGM thin films on porous anode supports by slurry spin coating for IT-SOFC. Rare Metals. 2014;34(11):797.
Kumar M, Srikanth S, Ravikumar B, Alex TC, Das SK. Synthesis of pure and Sr-doped LaGaO3, LaFeO3 and LaCoO3 and Sr, Mg-doped LaGaO3 for ITSOFC application using different wet chemical routes. Mater Chem Phys. 2009;113(2–3):803.
Zhou K, Chen H, Tian Q, Hao Z, Shen D, Xu X. Pd-containing perovskite-type oxides used for three-way catalysts. J Mol Catal A Chem. 2002;189(2):225.
Mostafavi E, Babaei A, Ataie A. Synthesis of nano-structured La0.6Sr0.4Co0.2Fe0.8O3 perovskite by co-precipitation method. J Ultrafine Grained Nanostruct Mater. 2015;48(1):45.
Tanaka H, Tan I, Uenishi M, Taniguchi M, Kimura M, Nishihata Y, Mizuki Ji. LaFePdO3 perovskite automotive catalyst having a self-regenerative function. J Alloy Compd. 2006; 408–412: 1071.
Gouadec G, Colomban P. Raman spectroscopy of nanomaterials: how spectra relate to disorder, particle size and mechanical properties. Prog Cryst Growth Charact. 2007;53(1):1.
Popa M, Frantti J, Kakihana M. Characterization of LaMeO3 (Me: Mn Co, Fe) perovskite powders obtained by polymerizable complex method. Solid State Ion. 2002;154–155:135.
Liu N, Guo T, Yan GQ, Zhu G, Guo HY. Magneto-electric behaviors of La0.67−x Nd x Sr0.33MnO3 system. Rare Met. 2015;. doi:10.1007/s12598-015-0538-x.
Andreasson J, Holmlund J, Knee CS, Käll M, Börjesson L, Naler S, Bäckström J, Rübhausen M, Azad AK, Eriksson S-G. Franck–Condon higher order lattice excitations in the LaFe1−x Cr x O3 (x = 0, 0.1, 0.5, 0.9, 1.0) perovskites due to Fe-Cr charge transfer effects. Phys Rev B. 2007;75(10):104302.
Gnezdilov V, Pashkevich YG, Yeremenko A, Lemmens P, Guntherodt G, Shiryaev S, Bychkov G, Barilo S. Phonon Raman scattering in LaMn1−x Co x O3 (x = 0, 0.2, 0.3, 0.4, and 1.0). Fiz Nizk Temp. 2003;29(11):1269.
Rt Shannon. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A Cryst. 1976;32(5):751.
Wang Z, Chen C, Feng C, Wang J, Zou B, Zhao M, Wu F. Synthesis, characterization and humidity sensitive properties of nanocrystalline LaCo x Fe1–x O3. Acta Phys Chim Sin. 2008;24(3):375.
Giang HT, Duy HT, Ngan PQ, Thai GH, Anh Thu DT, Thu DT, Toan NN. Effect of 3d transition metals on gas sensing characteristics of perovskite oxides LaFe1−x Co x O3. Anal Methods. 2013;5(16):4252.
Li J, Singh UG, Bennett JW, Page K, Weaver JC, Zhang JP, Proffen T, Rappe AM, Scott S, Seshadri R. BaCe1−x Pd x O3-δ (0 ≤ x ≤ 0.1): redox controlled ingress and egress of palladium in a perovskite. Chem Mater. 2007;19(6):1418.
Masliyah JH, Bhattacharjee S. Electrokinetic and Colloid Transport Phenomena. New Jersey: Wiley; 2006. 188.
Cushing BL, Kolesnichenko VL, O’Connor CJ. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev. 2004;104(9):3893.
Schweitzer GK, Pesterfield LL. The Aqueous Chemistry of the Elements. New York: Oxford University Press; 2009. 284.
Henrist C, Mathieu J-P, Vogels C, Rulmont A, Cloots R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J Cryst Growth. 2003;249(1):321.
Guo RS, Wei QT, Li HL, Wang FH. Synthesis and properties of La0.7Sr0.3MnO3 cathode by gel combustion. Mater Lett. 2006;60(2):261.
Porter DA, Easterling KE. Phase Transformations in Metals and Alloys (Revised Reprint). Boca Raton: CRC Press; 1992. 115.
Tang J, Zhu M, Zhong T, Hou Y, Wang H, Yan H. Synthesis of fine Pb(Fe0.5Nb0.5)O3 perovskite powders by coprecipitation method. Mater Chem Phys. 2007;101(2–3):475.
Reger D, Goode S, Ball D. Chemistry: Principles and Practice. Belmont: Cengage Learning; 2009. 17.
Wood SA. Experimental determination of the hydrolysis constants of Pt2+and Pd2+ at 25 °C from the solubility of Pt and Pd in aqueous hydroxide solutions. Geochim Cosmochim Acta. 1991;55(7):1759.
Yang Y, Chen O, Angerhofer A, Cao YC. Radial-position-controlled doping in CdS/ZnS core/shell nanocrystals. J Am Chem Soc. 2006;128(38):12428.
Romero M, Gómez RW, Marquina V, Pérez-Mazariego JL, Escamilla R. Synthesis by molten salt method of the AFeO3 system (A = La, Gd) and its structural, vibrational and internal hyperfine magnetic field characterization. Phys B. 2014;443:90.
Popa M, Frantti J, Kakihana M. Lanthanum ferrite LaFeO3+d nanopowders obtained by the polymerizable complex method. Solid State Ionics. 2002;154–155:437.
Iliev MN, Abrashev MV. Raman phonons and Raman Jahn-Teller bands in perovskite-like manganites. J Raman Spectrosc. 2001;32(10):805.
Martín-Carrón L, De Andres A, Martínez-Lope M, Casais M, Alonso J. Raman phonons as a probe of disorder, fluctuations, and local structure in doped and undoped orthorhombic and rhombohedral manganites. Phys Rev B. 2002;66(17):174303.
Venugopalan S, Dutta M, Ramdas A, Remeika J. Magnetic and vibrational excitations in rare-earth orthoferrites: a Raman scattering study. Phys Rev B. 1985;31(3):1490.
Koshizuka N, Ushioda S. Inelastic-light-scattering study of magnon softening in ErFeO3. Phys Rev B. 1980;22(11):5394.
Arora AK, Rajalakshmi M, Ravindran T, Sivasubramanian V. Raman spectroscopy of optical phonon confinement in nanostructured materials. J Raman Spectrosc. 2007;38(6):604.
Weber M, Kreisel J, Thomas PA, Newton M, Sardar K, Walton R. Phonon Raman scattering of RCrO3 perovskites (R = Y, La, Pr, Sm, Gd, Dy, Ho, Yb, Lu). Phys Rev B. 2012;85(5):054303.
Coplen TB. Atomic weights of the elements 1999. J Phys Chem Ref Data. 2001;30(3):701.
Idrees M, Nadeem M, Matiullah S, Shin TJ. Anomalous octahedral distortions in LaFe1−x Ni x O3. J Phys D Appl Phys. 2011;44(45):455303.
Yassin O, Alamri S, Joraid A. Effect of particle size and laser power on the Raman spectra of CuAlO2 delafossite nanoparticles. J Phys D Appl Phys. 2013;46(23):235301.
Acknowledgments
This work was financially supported by University of Tehran (No. 810729920/6/02) and Iran Nanotechnology Initiative Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varandili, S.B., Babaei, A. & Ataie, A. Characterization of B site codoped LaFeO3 nanoparticles prepared via co-precipitation route. Rare Met. 37, 181–190 (2018). https://doi.org/10.1007/s12598-016-0707-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0707-6