Abstract
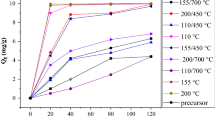
Nano-TiO2 was employed for the adsorption of gallium from aqueous solution in batch equilibrium experiments to investigate its adsorption properties. It was found that the adsorption efficiency of Ga(III) was more than 96% at pH 3.0. The adsorption capacities and rates of Ga(III) onto nano-TiO2 were evaluated as a function of solution concentration and temperature. The results were analyzed using the Langmuir adsorption isotherms. Adsorption isothermal data could be well interpreted by the Langmuir model. The mean energy of adsorption, 15.81 kJ·mol−1, was calculated from the D-R adsorption isotherm. The kinetic experimental data properly correlate with the pseudo-second-order kinetic model. The thermodynamic parameters for the process of adsorption have been estimated. The ΔH Ɵ and ΔG Ɵ values of gallium(III) adsorption on nano-TiO2 showed an endothermic and spontaneous nature of adsorption.
Similar content being viewed by others
References
Fergusson J.E., The Heavy Elements: Chemistry, Environmental Impact and Health Effects, Pergamon Press, Oxford, 1991: 1733.
Harris W.R. and Messori L., A comparative study of aluminum( III), gallium(III), indium(III), and thallium(III) binding to human serum transferring, Coord. Chem. Rev., 2002, 228(2): 237.
Chan C.V., Removal and recovery of gallium ion from solution by insoluble amphoteric starches, Appl. Polym. Sci., 1993, 50: 1733.
Hiroshi O. and Takeo S., Selective separation of Ga3+ through membrane impregnated with N-octadecanoyl-N-phenylhydroxylamine, J. Membr. Sci., 1995, 105(1–2): 43.
Puvvada G.V.K., Liquid-liquid extraction of gallium from Bayer process liquor using Kelex 100 in the presence of surfactants, Hydrometallurgy, 1999, 52(1): 9.
Chou W.L., Wang C.T., Yang K.C., and Huang Y.H., Removal of gallium(III) ions from acidic aqueous solution by supercritical carbon dioxide extraction in the green separation process, J. Hazard. Mater., 2008, 160(1): 6.
Anthemidis A.N., Zachariadis G.A., and Stratis J.A., Gallium trace on-line preconcentration/separation and determination using a polyurethane foam mini-column and flame atomic absorption spectrometry: Application in aluminum alloys, natural waters and urine, Talanta, 2003, 60(5): 929.
Chung N.H., Nishimoto J., Kato O., and Tabata M., Selective extraction of thallium in the presence of gallium, indium, bismuth and antimony by salting-out of an aqueous mixture of 2-propanol, Anal. Chim. Acta, 2003, 477(2): 243.
Hasegawa K., Shimada T., and Niitsu M., Solvent extraction of 3B group metal ions from hydrochloric acid with trioctylphosphine oxide, Inorg. Nucl. Chem., 1980, 42(10): 1487.
Reddy A.S., Solvent extraction separation of Tl/I/ from Tl/III/ with sulphoxides, J. Radionanal. Nucl. Chem., 1985, 94(4): 259.
Bénézeth P., Diakonov I., Pokrovski G., Dandurand J.L., and Schott J., Gallium solubility and aqueous speciation in hydrothermal solution (60–250°C): Experimental study and comparison with aluminum, Mineral. Mag., 1994, 58A(1): 71.
Aksoyoglu S., Sorption of U(VI) on granite, J. Radioanal. Nucl. Chem., 1989, 134(2): 393.
Memon S.Q., Bhanger M.I., Hasany S.M., and Khuhawar M.Y., Sorption behavior of impregnated Styrofoam for the removal of Cd(II) ions, Colloids Surf. A, 2006, 279: 142.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Zhu, Y., Li, H. et al. Kinetic and thermodynamic studies of adsorption of gallium(III) on nano-TiO2 . Rare Metals 29, 16–20 (2010). https://doi.org/10.1007/s12598-010-0003-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-010-0003-9