Abstract
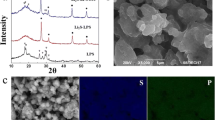
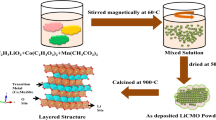
A new LiCoO2 recovery technology for Li-ion batteries was studied in this paper. LiCoO2 was peeled from the Al foil with dimethyl acetamide (DMAC), and then polyvinylidene fluoride (PVDF) and carbon powders in the active material were eliminated by high temperature calcining. Subsequently, Li2CO3, LiOH·H2O and LiAc·2H2O were added into the recycled powders to adjust the Li/Co molar ratio to 1.00. The new LiCoO2 was obtained by calcining the mixture at 850°C for 12 h in air. The structure and morphology of the recycled powders and resulting samples were studied by XRD and SEM techniques, respectively. The layered structure of LiCoO2 synthesized by adding Li2CO3 is the best, and it is found to have the best characteristics as a cathode material in terms of charge-discharge capacity and cycling performance. The first discharge capacity is 160 mAh·g−1 between 3.0–4.3 V. The discharge capacity after cycling for 50 times is still 145.2 mAh·g−1.
Similar content being viewed by others
References
Kim J., Byoug S.K., Lee J.G., Cho J., and Byung P.P.W., Differential voltage analyses of high-power, lithium-ion cells: I. Technique and application, J. Power Sources, 2005, 139(1–2): 289.
Li J.G., He X.M., and Zhao R.S., Electrochemical performance of SrF2-coated LiMn2O4 cathode material for Li-ion batteries, Trans. Nonferrous Met. Soc. China, 2007, 17(6): 1324.
Li Y., Michio T., and Wang B.F., A study on capacity fading of lithium-ion battery with manganese spinel positive electrode during cycling, Electrochem. Acta, 2006, 51(9): 3228.
Veronica P., Aintzane G., Izaskun G.M., Iratxe M., Miguel B., Oscar M., and Teofilo R., New freeze-drying method for LiFePO4 synthesis, J. Power Sources, 2007, 171(2): 879.
Wang Y.Q., Wang J.L., Yang J., and Nuli A.Y., High-rate LiFePO4 electrode material synthesis by a novel route from FePO4·4H2O, Adv. Funct. Mater., 2006, 16(2): 2135.
Zhang S.C., Qiu X.P., He Z.Q., Weng D.S., and Zhu W.T., Nanoparticled Li(Ni1/3Co1/3Mn1/3)O2 as cathode material for high-rate lithium-ion batteries, J. Power Sources, 2006, 153(1): 350.
Cho T.H., Shiosaki Y., and Noguchi H., Preparation and characterization of layered LiMn1/3Ni1/3Co1/3O2 as a cathode material by an oxalate co-precipitation method, J. Power Sources, 2006, 159(4): 1322.
Jinsik M., Jung Y.W., Lee J.Y., and Tak Y.S., Cobalt oxide preparation from waste LiCoO2 by electrochemical-hydrothermal method, J. Power Sources, 2002, 112(2): 639.
Zhang P.W., Yokoyama T., Itabashi O., Suzuki T.M., and Inoue K., Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries, Hydrometallurgy, 1998, 47(2–3): 259.
Michiael J.L., Recycling of lithium ion cells and batteries, J. Power Sources, 2001, 97–98(1): 736.
Lee C.K., and Rhee K.I., Preparation of LiCoO2 from spent lithium-ion batteries, J. Power Sources, 2002, 109(1): 17.
Cestaile M., Panero S., and Scrosati B., A laboratory-scale lithium-ion battery recycling process, J. Power Source, 2001, 92(1–2): 65.
Fang T., Duh J., and Sheen S., LiCoO2 cathode material coated with nano-crystallized ZnO for Li-ion batteries, Thin Solid Films, 2004, 469–470(1): 361.
Fey G.T., Lin Y.Y., and Kunar T.P., Enhanced cyclability and thermal stability of LiCoO2 coated with cobalt oxides, Surf. Coat. Technol., 2005, 191(1): 68.
Antoleini E., LiCoO2: formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties, Solid State Ionics, 2004, 170(1): 159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Zhong, S., Xiong, D. et al. Synthesis and electrochemical performances of LiCoO2 recycled from the incisors bound of Li-ion batteries. Rare Metals 28, 328–332 (2009). https://doi.org/10.1007/s12598-009-0064-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-009-0064-9