Abstract
Satellite cells are muscle stem cells capable of replenishing or increasing myonuclear number. It is postulated that a reduction in satellite cells may contribute to age-related sarcopenia. Studies investigating an age-related decline in satellite cells have produced equivocal results. This study compared the satellite cell content of young and aging soleus muscle in rat, using four different methods: dystrophin–laminin immunohistochemistry, MyoD immunohistochemistry, electron microscopy, and light microscopy of semi-thin sections. The absolute quantity of satellite cells increase with age, but satellite cell percentages were similar in young and aging soleus muscles. There were no differences in satellite cell quantity among MyoD immunohistochemistry, electron microscopy, and semi-thin sections. All three methods had significantly more satellite cells than with dystrophin–laminin immunohistochemistry. We conclude that satellite cell number does not decrease with age and postulate that satellite cell functionality may be responsible for age-related sarcopenia.
Similar content being viewed by others
Introduction
Skeletal muscle fibers are multinucleated, and each myonucleus governs a surrounding area of cytoplasm, called a nuclear domain [1, 2]. Myonuclei are post-mitotic, so new myonuclei are added via the activation of muscle-specific stem cells, called satellite cells. Satellite cells reside between the sarcolemma and the basal lamina of skeletal muscle fibers. They are established early in development and are required for postnatal growth of the fiber [3–6]. Upon activation, satellite cells proliferate and donate daughter nuclei to the muscle fiber.
Satellite cells become activated when adult muscle fibers are stimulated to undergo hypertrophy or reparative processes [7]. During hypertrophy, an increase in cytoplasm necessitates the addition of nuclei, in order to maintain a nuclear domain. It is postulated that without satellite cells, muscle fibers lose their ability to increase nuclear content and therefore cannot grow [6]. Conversely, muscle atrophy is associated with loss of myonuclei [8, 9]. Indeed, it appears apoptosis is at least partially responsible for age-related muscle atrophy [10]. It is unclear whether the quantity of satellite cells also declines with age. If so, the muscle’s ability to reverse age-related sarcopenia is severely compromised.
In one of the first quantitative studies done on satellite cells, Snow [11] proposed that satellite cell percentage is decreased with aging. Subsequent studies have either confirmed the original observation [12–14] or have found no change with aging [15–17]. Comparison of these data is confounded by the different animal strains, ages, and muscles used in the studies. Equally confounding, satellite cells are quantified in relation to myonuclear content, and this may vary depending upon a number of factors, such as skeletal muscle fiber type and size, and animal growth.
Several factors may be responsible for this variability, but it appears that the method for identifying satellite cells may be a major problem, especially since recent studies have suggested markedly different counts for satellite cells using different molecular markers [18]. Although electron microscopy is best, it is not feasible for all studies to use this method for identification.
Therefore, the purpose of this study was to investigate the effects of age on muscle stem cell content in the soleus muscle of male Fisher 344 rats. A second purpose of this study was to compare different methods of satellite cell identification. Soleus samples from young and old rats were independently compared by (1) a dystrohpin–laminin(DL) immunohistochemical method, (2) a MyoD immunohistochemical method, (3) electron microscopy (EM), and (4) light microscope (LM) analysis of semi-thin sections cut adjacent to the thin sections used for EM.
Materials and methods
Animals and muscle extraction
Young (5 months; N = 6) and old (24 months; N = 8) male Fisher 344 rats from the NIH Aging Colony (Harlan Labs, Indianapolis, IN) were anesthetized with xylazine and ketamine, and the soleus muscle was removed. The proximal portion was fixed for electron microscopy (see details below). The distal half of the muscle was prepared for immunohistochemistry. In brief, muscles were mounted for cross sectioning on tongue depressors using a mixture of tragacanth gum (Fisher Chemicals, Pittsburgh, PA) and OCT freezing compound (Sakura Finetek, Torrance, CA), frozen in methyl butane (Fisher Chemicals) chilled with liquid nitrogen, then stored in sealed vials at −80°C until sectioned. Serial cross sections were made at 6 μm thickness, mounted on poly-l-lysine-coated cover glasses, and stored at −40°C until processed. All procedures were approved by the Ohio University Institutional Animal Care and Use Committee.
Immunohistochemistry
To block endogenous peroxidase activity, unfixed 6 μm sections were first incubated for 10 min at room temperature in a 0.3% peroxidase in phosphate buffered saline (PBS) solution. Sections were then immunostained with monoclonal anti-dystrophin antibody (1:80 dilution in PBS; Sigma-Aldrich, St. Louis, MO; incubated with 1% BSA, 0.1% Tween-20 in PBS for 1.5 days), washed in washing buffer (PBS, 1% milk powder, 0.5% Tween-20) for 2 × 15 min and then a biotinylated secondary antibody (1:200 dilution, Vector Labs, Burlingame, CA; 60 min at 37°C) to label the sarcolemma. For blocking non-specific protein binding, the washing buffered contained skim milk power (1%). After processing for the avidin–biotin complex reaction (Vector Labs, Burlingame, CA; 45 min at RT), the same sections were then labeled with polyclonal anti-laminin (1:55 dilution in PBS; Santa Cruz Biotechnology, Santa Cruz, CA; incubated with 1% BSA, 0.1% Tween-20 in PBS for 2 h at RT) and then a biotinylated secondary antibody (1:200 dilution, Vector Labs, Burlingame, CA, for 60 min at RT) to identify the basal lamina. After washing with washing buffer (2 × 15 min), the sections were again processed for the avidin–biotin complex (ABC) reaction (Vector Labs, Burlingame, CA; 60 min), washed in PBS for 15 min, followed by an immunoperoxidase procedure using metal-enhanced diaminobenzidine (DAB; Pierce Chemical Company, Rockford, IL; 15 min). Next, the sections were lightly fixed with buffered 4% paraformaldehyde (2 min) and the nuclei lightly stained with Delafield’s hematoxylin (3 min) and then permanently mounted (dehydrated with alcohol: 70% ethanol, 2 min; 95% ethanol, 2 min 100% ethanol, 2 × 2 min; HemoD 2 × 2 min; mounted with mounting medium). This allowed the myonuclei (lying under the sarcolemma) to be distinguished from the satellite cells (lying between the sarcolemma and basal lamina; see Fig. 1).
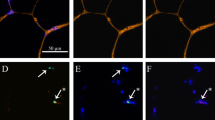
The procedures for MyoD immunohistochemistry were similar to the procedures for dystrophin immunohistochemistry. In brief, 6-μm sections were fixed in a 4% paraformaldehyde solution (10 min at 4°C), blocked in a 0.3% peroxidase in methanol solution (10 min), and then blocked with a solution of goat serum (1.5%) in PBS for 60 min. After washing in PBS, this was followed by a 60 min incubation in monoclonal anti-MyoD antibody (1:50 dilution in PBS at 37°C; Developmental Studies Hybridoma Bank). The sections were then incubated with a biotinylated secondary antibody (60 min), ABC reaction (60 min), and DAB reaction (10 min), as described above, including hematoxylin, washing, dehydration, hemoD and mounting with mounting medium. This procedure resulted in the accumulation of a dark precipitate in the nuclei containing MyoD (Fig. 2).
Cross-sectional area
Fiber cross-sectional areas (CSA) were measured using a video-capture microscope and NIH Image software (v 1.62). Cross-sectional area was determined from three random microscope frames per animal (~0.56 mm2, which corresponded to 3 × ~100 muscle fibers in each frame), or all fibers in the sample. Myonuclei were counted at ×1,000 magnification. The same histological preparation (dystrophin/laminin) was used for determination of CSA, satellite cells, and myonuclei. Fibers were not included if they were damaged or located at the edge of the muscle.
Electron microscopy
For electron microscopy, the muscles were fixed for several hours in dilute Karnovsky’s fixative (1.5% paraformaldehyde, 1% glutaraldehyde in 0.1 M cacodylate buffer) and diced into smaller pieces after at least 15 min of initial fixation. The segments were rinsed in buffer, post-fixed in 1% osmium tetroxide in cacodylate buffer, rinsed again, then stained en bloc with 1% aqueous uranyl acetate for 30–60 min. Tissues were dehydrated, cleared in limonene or propylene oxide, and embedded in a mixture of epon and araldite.
Blocks were trimmed for cross or longitudinal sections, and semi-thin sections were made at either 0.5 or 0.7 μm thickness, mounted on microscope slides, and stained with either 1% toluidine blue in 1% borax solution or a commercial epoxy resin staining solution (Electron Microscopy Sciences, Fort Washington, PA). Adjacent ultrathin sections were mounted on 150 mesh copper grids, stained with aqueous uranyl acetate and lead citrate, and examined with a Zeiss EM 109 or JEOL 1010 electron microscope.
Semi-thin sections (labeled as “light microscopy”) were analyzed under ×1,000 magnification, and satellite cells were differentiated from myonuclei by their thin, heterochromatic appearance. Often, the satellite cell nuclei were stained metachromatically (in the toluidine blue preparations) and were more purple than the pale myonuclei. This same type of staining was seen in endothelial cell nuclei between muscle fibers (Fig. 3).
For analysis by electron microscopy, a computer image of the thick section was used to identify each fiber being analyzed (Fig. 4). Each nucleus was examined at high magnification (×20,000) to insure correct identification of the satellite cell or myonucleus.
Different investigators independently analyzed and counted the nuclei and satellite cells. Each investigator analyzed the samples by electron microscopy, immunohistochemistry, or light microscopy of semi-thin sections; all counts with each method were determined independently of the others.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to compare the dependent variable (CSA, nuclei/fiber, satellite cells) across ages. A repeated measures ANOVA was used to compare techniques (EM, LM, DL, and MyoD) across ages. T tests were used for animal and muscle mass analyses. Significance was set at P ≤ 0.05. Where significance was found for main effects, Fisher’s least significant difference method was used for post-hoc analysis. Power calculations were determined for each dependent variable (Table 2).
Results
Animal and muscle characteristics
Body and muscle mass characteristics are shown in Table 1. Body mass and soleus muscle mass were significantly heavier in the aging animals, but the ratio of the soleus/body mass did not differ between age groups. The descriptive statistics found in Table 1 have been previously reported [19]. Furthermore, there were no significant differences in myofiber CSA in the soleus of aging compared to young animals (Table 2).
Satellite cells and myonuclei
The myonuclear content is presented as number of myonuclei per fiber for cross-sectional profile. There were significantly more myonuclei in the aging compared with young muscle fibers (P < 0.05; Table 2).
Satellite cells were identified using four different techniques: (1) electron microscopy; (2) dystrophin–laminin immunohistochemistry; (3) MyoD immunohistochemistry; (4) semi-thick sections for light microscopy. Satellite cells are reported as the percentage of total nuclei present in the muscle [satellite cells/(satellite cells + myonuclei)]. The percentage for each technique is shown in Table 3. The satellite cell percentages were similar between the preparations for EM, MyoD, and LM, in both young and aging soleus muscles (Table 3). However, there were significantly fewer satellite cells as assessed by the DL immunohistochemical technique compared to the quantities obtained via either EM, MyoD, or LM (Table 3). Regardless of the technique, satellite cell quantity did not significantly differ between age groups.
Discussion
The main purpose of this study was to examine age-related changes in satellite cell content in the soleus muscle. Studies examining the effects of aging on satellite cells have produced equivocal results. Mouse soleus muscle was compared at 8–10, 19–20, and 29–30 months, and the percentages of satellite cells were found by electron microscopy to be 4.6, 4.3, and 2.4, respectively [11]. Gibson and Schultz [12] also used electron microscopy to study rat EDL and soleus muscles at 1, 12, and 24 months of age. The EDL had a linear decrease in satellite cell percentage and in absolute number of satellite cells. The soleus had a decrease in satellite cell percentage, but had no change in absolute numbers between 1 and 24 months. In partial agreement with the findings of Gibson and Schultz, Shefer and colleagues [20] found a decrease in the absolute quantity of satellite cells in both the EDL and soleus muscles of aging mice.
Studies examining the effects of aging on satellite cells in humans have also produced equivocal results. Studies of human vastus lateralis muscle from 65 to 75 year olds [21] or 58–78 year olds [15] showed that satellite cell percentages do not decline with age, as measured by electron microscopy [21] or immunohistochemistry [15]. In contrast, Kadi et al. [13] measured a decreased satellite cell percentage in 70–83-year-old human tibialis anterior muscles using neural cell adhesion molecule (NCAM) for identification. The contrasting results may be due to either the older age group, use of a different muscle, or using NCAM. Sajko et al. [22] also used m-cad identification of satellite cells in human vastus lateralis muscle to make many measurements of the biopsies in young and old men. They concluded that the satellite cells per volume of muscle decline with age. In the present study, we found no difference in satellite cell percentage between the soleus of young and aging animals. However, the absolute number of satellite cells and myonuclei both increase with age in order for the satellite cell percentage to remain constant.
The aforementioned equivocal results may be due to differences in the methods used to identify satellite cells. The standard and most reliable means of identification is by electron microscopy. Quiescent satellite cells are identified by electron microscopy because they lie between the basal lamina and sarcolemma of the fibers; they have a high nucleus to cytoplasm ratio, few organelles, and a high ratio of nuclear heterochromatin to euchromatin [23]. Similarly, satellite cells may be quantified by their location, by staining the sarcolemma (dystrophin) and the basement membrane (laminin) with immunohistochemical methods. Nuclei located in this region belong to satellite cells. We chose to identify satellite cells by their location due to the complication that specific satellite cell markers brings. More recently, molecular markers such as transcription factors and cell adhesion molecules have been used to label the satellite cells (reviewed by [24, 25]). These techniques are appealing due to the time-intensive nature of the electron microscopy and dystrophin–laminin techniques. However, a complication with these molecular markers is that they may not be expressed in all stages of satellite cell activity, and all cells may not be labeled by antibodies to these markers. In considering the difference in labeling of satellite cells by different markers, it is likely that differences in activation are associated with differences in cell surface protein expression. Therefore, if active growth is occurring, the labeling may not accurately reflect the satellite cell content. However, we acknowledge the more recent labeling methods with a number of specific satellite cell markers are a valid addition to identification of satellite cells. The use of the molecular marker for myogenic regulatory factor MyoD was chosen due to the expression of MyoD in quiescent and activated satellite cells, which accurately identifies muscle specific satellite cells [26].
The second purpose of this study was to compare four different methods of quantifying satellite cells. We compared the two standard methods (electron microscopy and dystrophin–laminin) to one of the molecular markers (MyoD), and to an under-utilized technique (semi-thin epoxy sections). We found no difference in satellite cell percentage between young and aging rats, regardless of the quantification method. The satellite cell percentages were similar among the preparations for EM, MyoD, and LM, for both young and aging soleus muscles. Interestingly, regardless of the age, the DL preparations yielded significantly lower values (P < 0.001) than with the other three methods. The reason for this significant difference is unclear.
To the authors’ knowledge no other study has compared these three satellite cell identification techniques. Wernig et al. [27] isolated mouse muscle fibers and labeled resting satellite cells with m-cad, then embedded the fibers in epoxy resin for semi-thin and ultra-thin sectioning. Labeled satellite cells were identified in semi-thin sections, then ultra-thin sections were made and examined by electron microscopy to verify the presence of labeled satellite cells. All cells labeled were satellite cells, and no myonuclear labeling occurred. Their study showed that m-cad labeling is specific to quiescent satellite cells, but it did not determine whether all quiescent satellite cells were labeled. This was important because Beauchamp’s group, using CD-34, myf-5, and m-cad to label myogenic cells, had found a small number satellite cells were negative for all of these markers [28].
A number of other factors may also influence the acquisition of satellite cell percentage. The satellite cell percentage may change with or without a change in the absolute number of satellite cells if the myonuclear population is altered (reviewed in [29]). Muscle fiber atrophy may affect myonuclear quantity [8, 30, 31], although others have found no change in myonuclear numbers with atrophy [32, 33]. Hypertrophy or muscle growth is associated with an increased number of myonuclei [5, 15, 30, 34, 35]. However, a threshold of hypertrophy has been proposed to exist, after which myonuclear proliferation occurs. Until this threshold is attained, fiber growth without nuclear addition is thought to occur [36]. Finally, different muscles or procedures that alter fiber size may have no influence on the regulation of myonuclear domain [37].
In the present study, based on the power calculations, we found less than a 50% difference in satellite cell percentage between young and aging animals. This suggests that satellite cells may lose functionality rather than cell number, and this may underlie the loss of regenerative potential and lead to muscular atrophy with aging [38]. It is well known that older muscle does not function to the capacity of the young muscle and that the proliferation potential of satellite cells declines with age [39, 40]. The Notch signaling pathway may be involved in this (reviewed in [41]) as restoring this pathway in aged muscle will re-establish the muscle’s regenerative capacities [38, 42]. However, satellite cells are able to take part in regeneration and self-renewal even at older age [43, 44].
Our finding that satellite cell quantity does not decrease supports the hypothesis that extrinsic factors are at fault for the reduced regenerative capacity of satellite cells. Recent studies suggest factors in the systemic milieu may stimulate aged satellite cells. The Conboy laboratory has demonstrated that introducing serum from young mice to aging mice, via heterochronic parabiosis, was able to rescue the proliferative capacity of aging satellite cells [38]. Similarly, Shefer and colleagues have demonstrated that the regenerative capacity of aging satellite cells was increased when the local environment was supplemented with fibroblast growth factor [20]. A potential limitation of this study was the use of 24-month-old Fisher 344 rats as our aging cohort. In most rat strains, body mass continues to increase throughout the animal’s lifespan. However, the body mass of the Fisher 344 strain plateaus. This may explain why the present data are not in complete agreement with the data of Gibson and Schultz [12], which examined satellite quantity in the soleus of 24-month-old Sprague Dawley rats. We believe the growth plateau seen in the Fisher 344 strain is more applicable to human muscle growth. Furthermore, 24 months is the age at which NIH survival curves indicate a 50% rate of survival for this strain and is comparable to 80 years of age in humans [45]. According to the power analysis (Table 3), the sample size used in this study requires a substantial difference in the percent of satellite cells to detect significant differences. Thus, the light microscope approach can only detect 87% differences in percent, the MyoD and dystrophin–laminin immunohistochemistry require approximately 50%, and the gold-standard EM approach slightly less. While the changes required to detect significance are large, the values noted in each of the different methods employed here for analysis are similar.
Conclusion
Muscle atrophy is associated with weakness, which is a major contributor to impaired mobility and can lead to an increased risk of falling and fractures [46, 47]. Satellite cells are essential for muscle growth [6] and are likely to be involved in age-related sarcopenia. Previous research on the role of satellite cells with aging has produced equivocal results. This may be due to the methods used to identify satellite cells. We have used four different satellite cell identification methods to show that the percentage of satellite cells in rat soleus do not decrease with aging. Further, we demonstrated that semi-thin epoxy sections reliably identify satellite cell quantity if electron microscopy is not available. Satellite cell identification is comparable using semi-thin epoxy section or using anti-MyoD antibodies. Therefore, researchers may consider using semi-thin sections to check the reliability of their primary method of satellite cell identification.
Since satellite cells are important for regeneration and their quantities were stable across age groups, we postulate that satellite cells have decreased ability to respond to hypertrophic stimuli. It is also plausible that an age-related reduction in extrinsic factors is more responsible than any intrinsic deficiency in the aging satellite cell. Future research should now focus on determining whether age affects the activation or proliferative capacity of these satellite cells.
References
Hall ZW, Ralston E (1989) Nuclear domains in muscle cells. Cell 59:771–772
Pavlath GK, Rich K, Webster SG, Blau HM (1989) Localization of muscle gene products in nuclear domains. Nature 337:570–573
Kelly AM (1978) Perisynaptic satellite cells in the developing and mature rat soleus muscle. Anat Rec 190:891–904
Moss FP, Leblond CP (1971) Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170:421–436
Mozdziak PE, Schultz E, Cassens RG (1997) Myonuclear accretion is a major determinant of avian skeletal muscle growth. Am J Physiol 272:C565–C571
Rosenblatt JD, Yong D, Parry DJ (1994) Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 17:608–613
Bischoff R (1986) A satellite cell mitogen from crushed adult muscle. Dev Biol 115:140–147
Hikida RS, Van Nostran S, Murray JD, Staron RS, Gordon SE, Kraemer WJ (1997) Myonuclear loss in atrophied soleus muscle fibers. Anat Rec 247:350–354
Mitchell PO, Pavlath GK (2001) A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am J Physiol 281:C1706–C1715
Alway SE, Siu PM (2008) Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev 36:51–57
Snow MH (1977) The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res 185:399–408
Gibson MC, Schultz E (1983) Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 6:574–580
Kadi F, Charifi N, Denis C, Lexell J (2004) Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29:120–127
Renault V, Rolland E, Thornell L-E, Mouly V, Butler-Browne GS (2002) Distribution of satellite cells in the human vastus lateralis muscle during aging. Exp Gerontol 37:1511–1512
Hikida RS, Staron RS, Hagerman FC, Walsh S, Kaiser E, Shell S, Hervey S (2000) Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol 55A:B347–B354
Roth SM, Martel GF, Ivey FM, Leemer JT, Metter EJ, Hurley BF, Rogers MA (2000) Skeletal muscle satellite cell populations in healthy young and older men and women. Anat Rec 260:351–358
Schafer R, Zweyer M, Knauf U, Mundegar RR, Wernig A (2005) The ontogeny of soleus muscles in mdx and wild type mice. Neuromuscul Disord 15:57–64
Dedkov EI, Borisov AB, Wernig A, Carlson BM (2003) Aging of skeletal muscle does not affect the response of satellite cells to denervation. J Histochem Cytochem 51:853–863
Brooks NE, Schuenke MS, Hikida RS (2009) Ageing influences myonuclear domain size differently in fast and slow skeletal muscle of rats. Acta Physiol 197:55–63
Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z (2006) Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294:50–66
Roth GS, Ingram DK, Black A, Lane MA (2000) Effects of reduced energy intake on the biology of aging: the primate model. Eur J Clin Nutr 54(Suppl 3):S15–S20
Sajko S, Kubinova L, Cvetko E, Kreft M, Wernig A, Erzen I (2004) Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J Histochem Cytochem 52:179–185
Schultz E (1976) Fine structure of satellite cells in growing skeletal muscle. Am J Anat 147:49–70
Charge S, Rudnicki MA (2003) Fusion with the fused: a new role for interleukin-4 in the building of muscle. Cell 113:422–423
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Zammit PS (2008) All muscle satellite cells are equal, but are some more equal than others? J Cell Sci 121:2975–2982
Wernig A, Bone M, Irintchev A, Schafer R, Cullen MJ (2004) M-cadherin is a reliable marker of quiescent satellite cells in mouse skeletal muscle. Basic Appl Myol 14:161–168
Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1234
Allen DL, Roy RR, Edgerton VR (1999) Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22:1350–1360
Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR (1997) Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 273:C579–C587
Allen DL, Yasui W, Tanaka T, Ohira Y, Nagaoka S, Sekiguichi C, Hinds WE, Roy RR, Edgerton VR (1996) Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J Appl Physiol 81:145–151
Ohira Y, Yoshinaga T, Ohara M, Nonaka I, Yoshioka T, Yamashita-Goto K, Shenkman BS, Kozlovskaya IB, Roy RR, Edgerton VR (1999) Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J Appl Physiol 87:1776–1785
Wada KI, Takahashi H, Katsuta S, Soya H (2002) No decrease in myonuclear number after long-term denervation in mature mice. Am J Physiol 283:484–488
Kadi F, Thornell L-E (2000) Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113:99–103
McCall GE, Allen DL, Linderman JK, Grindeland RE, Roy RR, Mukku VR, Edgerton VR (1998) Maintenance of myonuclear domain size in rat soleus after overload and growth hormone/IGF-1 treatment. J Appl Physiol 84:1407–1412
Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL (2004) The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558:1005–1012
Verheul AJ, Mantilla CB, Zhan WZ, Bernal M, Dekhuijzen PN, Sieck GC (2004) Influence of corticosteroids on myonuclear domain size in the rat diaphragm muscle. J Appl Physiol 97:1715–1722
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764
Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE (2004) Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol 97:1082–1090
Schultz E, Lipton BH (1982) Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev 20:377–383
Luo D, Renault VM, Rando TA (2005) The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol 16:612–622
Conboy IM, Conboy MJ, Smythe GM, Rando TA (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302:1575–1577
Bockhold KJ, Rosenblatt JD, Partridge TA (1998) Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve 21:173–183
Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA (2007) A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25:885–894
Rice KM, Linderman JK, Kinnard RS, Blough ER (2005) The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology 6:335–343
Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GS, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ (1994) Exercise training and nutritional supplementation for physical frailty in very elderly people. New Engl J Med 330:1769–1775
Porter MM, Vandervoort AA, Lexell J (1995) Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 5:129–142
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Brooks, N.E., Schuenke, M.D. & Hikida, R.S. No change in skeletal muscle satellite cells in young and aging rat soleus muscle. J Physiol Sci 59, 465–471 (2009). https://doi.org/10.1007/s12576-009-0058-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-009-0058-2