Abstract
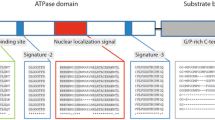
Heat shock proteins (HSPs) are proteins that are expressed more strongly when the cells are exposed to physiological and stressful conditions. In this study, the full-length cDNAs of heat shock proteins 40 (MjHSP40), 70 (MjHSP70) and 90 (MjHSP90) were cloned from kuruma shrimp Marsupenaeus japonicus. The open reading frames (ORFs) of the cDNA clones have lengths of 1,191, 1,959 and 2,172 bp and encode 396, 652 and 723 amino acid residues, respectively. The predicted MjHSP40 amino acid sequence contains a J domain, a glycine/phenylalanine-rich region, and a central domain containing four repeats of a CxxCxGxG motif, indicating that it is a type I HSP40 homolog. The signature sequences of the HSP70 and HSP90 gene families are conserved in the MjHSP70 and MjHSP90 amino acid sequences. The deduced amino acid sequences of MjHSP70 and MjHSP90 share high identity with previously reported shrimp HSP70s and HSP90s, respectively. The expression of MjHSP90 mRNA increased at 32°C. Additionally, the expressions of MjHSP40, MjHSP70 and MjHSP90 mRNAs increased in defense-related tissues (i.e., hemocytes and lymphoid organ) when the shrimp were challenged with white spot syndrome virus.




Similar content being viewed by others
References
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Nover L, Scharf KD (1997) Heat stress proteins and transcription factors. Cell Mol Life Sci 53:80–103
Qiu XB, Shao YM, Miao S, Wang L (2006) The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci 63:2560–2570
Cyr DM, Langer T, Douglas MG (1994) DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci 19:176–181
Cheetham ME, Caplan AJ (1998) Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones 3:28–36
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C (2001) The sequence of the human genome. Science 291:1304–1351
Hill RB, Flanagan JM, Prestegard JH (1995) 1H and 15 N magnetic resonance assignments, secondary structure, and tertiary fold of Escherichia coli DnaJ(1–78). Biochemistry 34:5587–5596
Qian YQ, Patel D, Hartl FU, McColl DJ (1996) Nuclear magnetic resonance solution structure of the human Hsp40 (HDJ-1) J-domain. J Mol Biol 260:224–235
Sahi C, Craig EA (2007) Network of general and specialty J protein chaperones of the yeast cytosol. Proc Natl Acad Sci USA 104:7163–7168
Jin Y, Awad W, Petrova K, Hendershot LM (2008) Regulated release of ERdj3 from unfolded proteins by BiP. EMBO J 27:2873–2882
Petrova K, Oyadomari S, Hendershot LM, Ron D (2008) Regulated association of misfolded endoplasmic reticulum lumenal proteins with P58/DNAJc3. EMBO J 27:2862–2872
Summers DW, Douglas PM, Ramos CH, Cyr DM (2009) Polypeptide transfer from Hsp40 to Hsp70 molecular chaperones. Trends Biochem Sci 34:230–233
Brychzy A, Rein T, Winklhofer KF, Hartl FU, Young JC, Obermann WM (2003) Cofactor Tpr2 combines two TPR domains and a J domain to regulate the Hsp70/Hsp90 chaperone system. EMBO J 22:3613–3623
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92:351–366
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Kiang JG, Tsokos GC (1998) Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 80:183–201
Flaherty KM, DeLuca-Flaherty C, McKay DB (1990) Three-dimensional structure of the ATPase fragment of a 70 K heat-shock cognate protein. Nature 346:623–628
Picard D (2002) Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci 59:1640–1648
Buchner J (1999) Hsp90 & Co.—a holding for folding. Trends Biochem Sci 24:136–141
Pratt WB, Toft DO (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 228:111–133
Luan W, Li F, Zhang J, Wen R, Li Y, Xiang J (2010) Identification of a novel inducible cytosolic Hsp70 gene in Chinese shrimp Fenneropenaeus chinensis and comparison of its expression with the cognate Hsc70 under different stresses. Cell Stress Chaperones 5:83–93
Cesar JR, Yang J (2007) Expression patterns of ubiquitin, heat shock protein 70, α-actin and β-actin over the molt cycle in the abdominal muscle of marine shrimp Litopenaeus vannamei. Mol Reprod Dev 74:554–559
Wu R, Sun L, Lei M, Xie ST (2008) Molecular identification and expression of heat shock cognate 70 (HSC70) in the Pacific white shrimp Litopenaeus vannamei. Mol Biol (Mosk) 42:265–274
Lo WY, Liu KF, Liao IC, Song YL (2004) Cloning and molecular characterization of heat shock cognate 70 from tiger shrimp (Penaeus monodon). Cell Stress Chaperones 9:332–343
Chuang KH, Ho SH, Song YL (2007) Cloning and expression analysis of heat shock cognate 70 gene promoter in tiger shrimp (Penaeus monodon). Gene 405:10–18
Li F, Luan W, Zhang C, Zhang J, Wang B, Xie Y, Li S, Xiang J (2009) Cloning of cytoplasmic heat shock protein 90 (FcHSP90) from Fenneropenaeus chinensis and its expression response to heat shock and hypoxia. Cell Stress Chaperones 14:161–172
Jiang S, Qiu L, Zhou F, Huang J, Guo Y, Yang K (2009) Molecular cloning and expression analysis of a heat shock protein (Hsp90) gene from black tiger shrimp (Penaeus monodon). Mol Biol Rep 36:127–134
Wu LT, Chu KH (2008) Characterization of heat shock protein 90 in the shrimp Metapenaeus ensis: evidence for its role in the regulation of vitellogenin synthesis. Mol Reprod Dev 75:952–959
Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, Bork P (2006) SMART 5: domains in the context of genomes and networks. Nucleic Acids Res 34:D257–D260
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the \(2^{{ - \rm\Updelta \rm\Updelta \it{C}_{{\text T}} }} \) method. Methods 25:402–408
Lindenstrøm T, Secombes CJ, Buchmann K (2004) Expression of immune response genes in rainbow trout skin induced by Gyrodactylus derjavini infections. Vet Immunol Immunopathol 97:137–148
Abramoff MD, Magelhaes PJ, Ran SJ (2004) Image processing with ImageJ. Biophoto Int 11:36–42
Welch WJ (1993) How cells respond to stress. Sci Am 268:56–64
Chouchane L, Bowers S, Sawasdikosol S, Simpson RM, Kindt TJ (1994) Heat shock proteins expressed on the surface of human T cell leukemia virus type I-infected cell lines induce autoantibodies in rabbits. J Infect Dis 169:253–259
Lathangue NB, Latchman DS (1988) A cellular protein related to heat shock protein 90 accumulates during herpes-simplex-virus infection and is overexpressed in transformed cells. Exp Cell Res 178:169–179
Donati YRA, Slosman DO, Polla BS (1990) Oxidative injury and the heat shock response. Biochem Pharmacol 40:2571–2577
Fincato G, Polentarutti N, Sica A, Mantovab A, Collotti F (1991) Expression of a heat inducible gene of the Hsp70 family in human myelomonocytic cells: regulation by bacterial products and cytokines. Blood 77:579–586
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Develop 12:3788–3796
Parsell DA, Lindquist S (1993) The function of heat shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Ann Rev Genetics 27:437–496
Szyperski T, Pellecchia M, Wall D, Georgopoulos C, Wuthrich K (1994) NMR structure determination of the Escherichia coli DnaJ molecular chaperone: secondary structure and backbone fold of the N-terminal region (residues 2–108) containing the highly conserved J domain. Proc Natl Acad Sci USA 91:11343–11347
Corsi AK, Schekman R (1997) The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. J Cell Biol 137:1483–1493
Young JC, Agashe VR, Siegers K, Hartl FU (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5:781–791
Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL (2005) Not all J domains are created equal: implications for the specificity of Hsp40–Hsp70 interactions. Protein Sci 14:1697–1709
Rudiger S, Scneider-Mergener J, Bukau B (2001) Its substrate specificity characterizes the DnaJ co-chaperones as a scanning factor for the DnaK chaperone. EMBO 20:1042–1050
Fan C-Y, Lee S, Ren H-Y, Cyr DM (2004) Exchangeable chaperone modules contribute to specification of type I and type II Hsp40 cellular function. Mol Biol Cell 15:761–773
Park K, Kwak IS (2008) Characterization of heat shock protein 40 and 90 in Chironomus riparinus larvae: effects of di(2-ethylhexayl) phthalate exposure on gene expression. Chemosphere 74:89–95
Liu J, Yang W-J, Zhu X-J, Karouna-Renier NK, Rao RK (2004) Molecular cloning and expression of HSP70 genes in the prawn, Macrobrachium rosenbergii. Cell Stress Chaperones 9:313–323
Wang B, Li F, Dong B, Zhang X, Xiang J (2006) Discovery of the genes in response to white spot syndrome virus (WSSV) infection in Fenneropenaeus chinensis through cDNA microarray. Mar Biotechnol (NY) 8:491–500
Gross PS, Bartlett TC, Browdy CL, Chapman RW, Warr GW (2001) Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific White Shrimp, Litopenaeus vannamei, and the Atlantic White Shrimp, L. setiferus. Dev Comp Immunol 25:565–577
Acknowledgments
This study was supported in part by grants from the Ministry of Agriculture, Forestry, and Fisheries of Japan and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the JSPS-NRCT Asian Core University program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danwattananusorn, T., Fagutao, F.F., Shitara, A. et al. Molecular characterization and expression analysis of heat shock proteins 40, 70 and 90 from kuruma shrimp Marsupenaeus japonicus . Fish Sci 77, 929–937 (2011). https://doi.org/10.1007/s12562-011-0394-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0394-z