Abstract
Talin is a large cytoskeletal protein (2541 amino acid residues) which plays a key role in integrin-mediated events that are crucial for cell adhesion, migration, proliferation and survival. This review summarises recent work on the structure of talin and on some of the structurally better defined interactions with other proteins. The N-terminal talin head (approx. 50 kDa) consists of an atypical FERM domain linked to a long flexible rod (approx. 220 kDa) made up of a series of amphipathic helical bundle domains. The F3 FERM subdomain in the head binds the cytoplasmic tail of integrins, but this interaction can be inhibited by an interaction of F3 with a helical bundle in the talin rod, the so-called “autoinhibited form” of the molecule. The talin rod contains a second integrin-binding site, at least two actin-binding sites and a large number of binding sites for vinculin, which is important in reinforcing the initial integrin–actin link mediated by talin. The vinculin binding sites are defined by hydrophobic residues buried within helical bundles, and these must unfold to allow vinculin binding. Recent experiments suggest that this unfolding may be mediated by mechanical force exerted on the talin molecule by actomyosin contraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Integrins are a large family of cell-surface type 1 transmembrane adhesion receptors that mediate both cell–cell and cell–extracellular matrix (ECM) interactions. Integrins also have the unusual ability to support bidirectional signalling. Thus, the binding of integrins to the ECM potentiates the ability of growth factors to activate intracellular pathways that regulate cell proliferation, survival and migration ("outside-in signalling"), whilst the affinity of integrins for ECM proteins can be regulated from within the cell, the so-called "inside-out signalling" (Hynes 2002). Integrins are heterodimers of α and β subunits, each containing a large extracellular domain (approx. 80–150 kDa), a single transmembrane α-helix and a short, largely unstructured, cytoplasmic domain or “tail” of 10–70 residues. In mammals, one of 18 α-subunits interacts with one of eight β-subunits to form 24 distinct integrins, each with specific but overlapping functions (Hynes 2002).The current state of knowledge of integrins and integrin signalling is the topic of a recent special issue of the Journal of Cell Science (vol 122, issue 2, 2009).
Cell adhesion to the ECM is fundamental to the development of multi-cellular organisms and involves the coordinated assembly and disassembly of integrins into complexes called focal adhesions. In these complexes, the internal tails of integrin β-subunits are typically linked to the actin cytoskeleton via cytoplasmic proteins with scaffolding, adaptor, regulatory and mechanotransduction functions (Legate and Fassler 2009; Zaidel-Bar et al. 2007). An analysis of the proteins that are currently known to assemble into focal adhesions identified 90 core components physically located at adhesion sites (Zaidel-Bar et al. 2007), and 42 proteins have been identified that reportedly bind just to the cytoplasmic tails of β-integrins (Legate and Fassler 2009). Among these proteins, the cytoskeletal protein talin has been shown to play a pivotal role in integrin-mediated events (Critchley 2009; Critchley and Gingras 2008). Talin promotes integrin clustering (Cluzel et al. 2005) and the switching of integrins from a low to high affinity state (Calderwood 2004; Harburger and Calderwood 2009; Tadokoro et al. 2003), although this also requires the kindlin family of proteins (Larjava et al. 2008). Talin also provides a direct link between integrins and the actin cytoskeleton (Jiang et al. 2003; Zhang et al. 2008) and acts as a scaffold for the recruitment of other proteins, such as vinculin (Ziegler et al. 2006). In this review we focus on recent studies of the structure and biophysics of talin, the results of which are beginning to throw light on the relationship between the structural and dynamic properties of this molecule and its function in the cell.
The domain structure of talin
There are two talin genes in vertebrates (Monkley et al. 2001; Senetar and McCann 2005). These code for talin1 and talin2, respectively, which are both large proteins (2541 amino acids; approx. 270 kDa) consisting of a globular N-terminal head (approx. 50 kDa) and a large flexible C-terminal rod (approx. 220 kDa) (Fig. 1). The talin head contains a FERM domain (residues 86–400) composed of F1, F2 and F3 domains. While initial attempts to crystallise the entire FERM domain have been unsuccessful to date, probably due to a large unstructured loop in F1 (Goult et al., in preparation), the crystal structure of the F2F3 fragment (Garcia-Alvarez et al. 2003) confirmed its structural similarity to the corresponding part of other FERM domains, with the F3 domain having a phosphotyrosine binding (PTB)-like fold (Fig. 1). The 85 amino acids preceding F1 were initially ignored, but recent nuclear magnetic resonance (NMR) studies show that they constitute a folded domain, the F0 domain, which has a ubiquitin-like fold (Goult et al., in preparation) as does the F1 domain. The structure of an F0F1 double domain construct with the flexible F1 loop removed shows a well-defined and rather rigid interface between the two domains (Goult et al., in preparation) (Fig. 1).
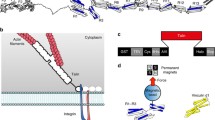
Diagrammatic representation of the domain structure of talin, showing the location of the binding sites identified for other proteins, including integrins, actin and vinculin. Helices which bind vinculin are shown in red. Cartoon representations are shown for those domains whose structure has been determined (see text for references)
The talin rod contains 62 helices that are organised into a series of helical bundles followed by a single C-terminal helix that forms an antiparallel homodimer (Gingras et al. 2008) (Fig. 1). Given the flexibility of the rod, the relative orientation of the two talin molecules within the dimer is uncertain. The rod starts with a five-helix bundle (residues 482–655) (Papagrigoriou et al. 2004); the crystal structure of talin 482–789 shows that residues 656–789 form a four-helix bundle that packs tightly against the talin 482–655 five-helix bundle in a staggered arrangement which is stabilised by an extensive hydrophobic interface (Papagrigoriou et al. 2004). The C-terminal half of the rod is also made up of a series of five-helix bundles (Cheung et al. 2009; Gingras et al. 2006, 2008,2009; Goult et al. 2009), but in this region a relatively flexible interface and end-to-end packing between the helical bundles seems to be typical. The central part of the rod also contains four- and five-helix bundles, but their packing with respect to one another has not yet been established with certainty. Biophysical and electron microscopy (EM) studies suggest that talin exists in several different conformational states. Thus, sedimentation equilibrium experiments show that it can exist as both monomers and dimers (Molony et al. 1987). Sedimentation velocity, gel filtration and EM studies indicate that it is globular in low salt buffers, whereas it is a flexible elongated molecule (approx. 60 nm in length) in 0.15 M salt and appears to have 10–12 globular domains (Molony et al. 1987; Winkler et al. 1997). The globular state appears to be dependent on a head/rod interaction, and recent data have provided insights into the structural basis of this interaction (Goksoy et al. 2008; Goult et al. 2009).
Protein partners of talin
The role of talin as an adaptor protein in linking integrins to the cytoskeleton and to signalling pathways in the cell is clearly indicated by the large number of proteins that have been shown to interact with talin (Critchley 2009; Critchley and Gingras 2008; Zaidel-Bar et al. 2007). Only a small number of the structurally better characterised interactions will be considered here.
The talin F3 FERM domain binds the cytoplasmic domains of β-integrin subunits (Calderwood et al. 2002) and the hyaluronan receptor layilin (Borowsky and Hynes 1998; Wegener et al. 2008) as well as the C-terminal region of PIPKIγ90 (Barsukov et al. 2003; de Pereda et al. 2005; Di Paolo et al. 2002; Ling et al. 2002), a splice variant of phosphatidylinositol(4)- phosphate-5-kinase type Iγ which regulates the assembly of focal adhesions. The talin head also contains an F-actin binding site (Lee et al. 2004) and binds acidic phospholipids (e.g. Dietrich et al. 1993; Goldmann et al. 1995; Niggli et al. 1994).
The talin rod contains a second integrin binding site, IBS2 (Gingras et al. 2009; Moes et al. 2007; Rodius et al. 2008; Tremuth et al. 2004; Xing et al. 2001) and at least two actin-binding sites (Hemmings et al. 1996), the best characterised of which is at the C-terminus (Gingras et al. 2008; McCann and Craig 1997; Senetar et al. 2004; Smith and McCann 2007). Importantly, the rod also contains multiple binding sites for vinculin (Gingras et al. 2005), which itself has numerous binding partners, including F-actin (Ziegler et al. 2006); the “cross-linking” of talin to actin by vinculin has been proposed to stabilise the initial weak integrin/talin/F-actin complexes (Bakolitsa et al. 2004).
Integrin binding
As noted above, the talin F3 domain has a PTB-like fold; canonical ligands of PTB domains are peptides containing an NPxY-like motif sequence. The short cytoplasmic tails of β-integrin subunits contain two such motifs, and the talin F3 FERM domain selectively binds to the membrane proximal to these, 744NPLY747 (Campbell and Ginsberg 2004). The structure of F3 bound to residues 739WDTANNPLYDEA750 of the β3-integrin tail (Garcia-Alvarez et al. 2003) shows that the integrin peptide interacts predominantly with the hydrophobic surface on strand S5 of the F3 domain; W739 inserts into a pocket on the talin surface, with the subsequent residues forming a β-strand that extends the sheet formed by strands 5–7 of the F3 domain. Y747 projects into an acidic pocket in F3, whereas the equivalent region in those PTB domains that bind phosphotyrosine is strongly basic.
More recent studies have shown that F3 also interacts with the membrane-proximal helix of the β3-tail; F727 and F730, which are on the same face of this helix, bind to a hydrophobic pocket in F3 made up of the flexible loop between β-strands 1 and 2 (Wegener et al. 2007). A mutation of either F727 or F730 in β3-integrin, or of the interacting residues in F3 (notably L325), markedly reduced the activation of αIIbβ3 integrin (Wegener et al. 2007). These authors propose that talin F3 initially binds to β3-integrin 744NPxY747 and subsequently to the membrane-proximal helix, thereby breaking the salt bridge between the α- and β-integrin tails which locks the integrin in a low affinity state. This hypothesis is supported by results from bimolecular fluorescence complementation studies in cells in which a talin L325R mutant was recruited to αIIbβ3-integrin tails but was unable to activate the integrin (Watanabe et al. 2008). However, talin F3 alone is not sufficient to activate β1-integrins, and the F0 and F1 domains are also required (Bouaouina et al. 2008).
A variety of experiments indicate that the integrin binding sites in full-length talin are masked (Calderwood 2004; Martel et al. 2001), and recent NMR studies show that this is due to an interaction between the talin head and rod (Goksoy et al. 2008). Talin F3 was shown to bind to a talin rod fragment spanning residues 1654–2344, partially masking the binding site in F3 for the membrane-proximal helix of the β3 integrin tail. This rod fragment contained higher affinity (residues 1654–1848) and lower affinity (residues 1984–2344) F3 binding sites. The domain boundaries of the region containing the high affinity site have now been defined structurally (Goult et al. 2009). The domain (residues 1655–1822) forms a five-helix bundle, and the positively charged integrin activation loop in F3 binds to a cluster of acidic residues, predominantly on helix 4, masking the binding site for the β3 integrin tail. The interaction is also expected to inhibit sterically the association of the talin FERM domain with the membrane. These results establish a structural basis for the “autoinihibited” form of talin. The mechanisms which disrupt this interaction to activate talin require further investigation, although the small GTPase Rap1 and its binding partner RIAM have been shown to play a key role in talin activation (Han et al. 2006; Lee et al. 2009; Watanabe et al. 2008), and PIPKIγ90 and PIP2 have also been implicated (Goksoy et al. 2008; Ling et al. 2002; Ling et al. 2003; Martel et al. 2001).
There is evidence for a second integrin binding site (IBS2) towards the C-terminal end of the talin rod (Tremuth et al. 2004; Xing et al. 2001), which has been suggested to correspond to a single predicted helix (Moes et al. 2007; Rodius et al. 2008). A crystal structure has recently been reported for the region of the rod containing IBS2: mouse talin residues 1974–2293 (Gingras et al. 2009) comprises two five-helix bundles—“IBS2-A” (1974–2139) and “IBS2-B” (2140–2293)—connected by a continuous helix. The single helix previously identified as IBS2 corresponds to helix 4 in IBS2-A. However, tight integrin binding and targeting to focal adhesions appears to require both the IBS2-A and IBS2-B domains (Gingras et al. 2009), and it remains to be established exactly how this region of the rod binds to integrins.
The C-terminal actin binding site in the rod
Talin has binding sites for actin in the head (the F2F3 region) and in two distinct regions of the rod (Critchley 2009; Hemmings et al. 1996). The structure of the C-terminal actin binding site of talin1 (Gingras et al. 2008) comprises a five-helix bundle (residues 2300–2482), similar to the homologous region of the HIP1R protein (Brett et al. 2006); this domain is now referred to as a THATCH domain. The actin binding site maps to a conserved hydrophobic surface on helices 3 and 4 of the five-helix bundle that is flanked by basic residues. Interestingly, helix 1, which packs against the opposite face of the bundle, negatively regulates actin binding (Senetar et al. 2004). Deletion of this helix or the mutation of residues involved in its packing against the remainder of the bundle significantly increases actin binding (Gingras et al. 2008; Senetar et al. 2004), and there appear to be significant structural changes upon removal of the helix (Gingras et al. 2008).
The C-terminal helix of talin forms an antiparallel coiled-coil dimer (Gingras et al. 2008). F-actin only binds efficiently to the THATCH dimer, residues 2300–2541 (Gingras et al. 2008; Smith and McCann 2007), and residues on one face of the dimerisation helix may also contribute to actin binding (Gingras et al. 2008). SAXS experiments indicate that the THATCH dimer adopts an elongated structure in solution, and the high-resolution structures of the five-helix bundle and dimerisation domain can be readily modeled within the SAXS envelope. EM studies show that the THATCH dimer binds to three actin monomers along the long pitch of the same actin filament (Gingras et al. 2008); it does not cross-link F-actin—presumably, the actin-bundling activity of talin is explained by the presence of the other actin binding sites in talin.
Binding of vinculin to the talin rod
Vinculin is a 116-kDa actin-binding protein that is localised in focal adhesions. It is made up of a globular head linked to a tail domain by a proline-rich region, and interaction sites for numerous binding partners have been mapped onto all three regions of the protein (Bakolitsa et al. 2004; Borgon et al. 2004; Ziegler et al. 2006). All of the well-characterised ligand-binding sites in vinculin (including that for talin) are masked by an intramolecular interaction between the vinculin head and tail domains (Bakolitsa et al. 2004; Borgon et al. 2004; Cohen et al. 2005; Izard et al. 2004; Johnson and Craig 1994, 1995a, b), and the molecule is thought to exist in an equilibrium between active and inactive states.
Initial studies identified at least three vinculin binding sites (VBSs) in the talin rod, and these were localised to three short peptide sequences (VBS1–3), each corresponding to a single predicted α-helix (Bass et al. 1999). A systematic analysis of the binding of vinculin to peptides corresponding to each of the 62 helices of the rod revealed as many as 11 VBSs (Gingras et al. 2005), although it is clear that not all of these are available in the intact protein in vitro (Patel et al. 2006).
The talin-binding site in vinculin has been localised to residues 1–258 within the vinculin head (Bass et al. 2002), a helical domain (Vd1) which binds the vinculin tail (Vt) with high affinity (Gilmore and Burridge 1996; Johnson and Craig 1994). Binding the talin VBS3 peptide induces a marked conformational change in Vd1 that displaces Vt, and the VBS3 peptide itself sits in a hydrophobic groove formed predominantly by helices 1 and 2 of Vd1 (Izard et al. 2004). Similar results have been reported for Vd1 in complex with other VBSs (Fillingham et al. 2005; Gingras et al. 2005; Papagrigoriou et al. 2004).
The structure of the N-terminal part of the talin rod (residues 482– 655) shows that it consists of a 5-helix bundle in which helix 4 is equivalent to VBS1 (Papagrigoriou et al. 2004). The key vinculin-binding determinants are five hydrophobic residues (L608, A612, L615, V619 and L623) on one face of the VBS1 helix which are normally buried within the hydrophobic core of the five-helix bundle. Comparison of the structure of the Vd1/VBS1 complex with that of talin 482–655 shows that Vd1 helices 1–4 occupy the equivalent positions in relation to the VBS1 helix as do helices 1, 5, 2 and 3 in the talin 482–655 five-helix bundle, and key hydrophobic contacts are maintained by interactions with similar side chains in Vd1. Thus, the VBS1 helix (helix 4) is extracted from its own five-helix bundle and forms an equivalent five-helix bundle with the four helices of Vd1 (Papagrigoriou et al. 2004) (Fig. 2).
a Topological equivalence of vinculin binding site (VBS)1 helix (red) within the talin 482–655 structure (blue) and the Vd1/VBS1 complex structure (green). b End-on view of the superposed helices of talin 482–655 (blue) and the Vd1/VBS1 complex (green)—with the VBS1 helix in red in each case. Data in a and b are from Papagrigoriou et al. (2004). c Electron paramagnetic resonance (EPR) spectra of spin-labelled talin 1843–1973. Top Room temperature (298 K) spectra of a sample spin-labelled at residue 1927. In the absence of Vd1 (black line), the line shape indicates a moderately mobile spin label with signs of tertiary interaction. After the addition of Vd1, the mobility increases (red line), showing a line shape corresponding to an opened, disordered structure. Bottom Measured (solid) and calculated (dashed) low-temperature (155 K) spectra of a sample spin-labelled at residues 1887 and 1927 in the absence (black) and presence (red) of Vd1. The theoretical spectra were calculated using a Gaussian distance distribution of 4.0 Å and a mean distance of <9 Å in the first and >20 Å in the second case, indicating a dramatic increase of the distance between the two labeled positions on binding to Vd1. Data from Gingras et al. (2006)
This striking observation implies that the helical bundle of talin 482–655 must unfold to allow the vinculin binding sequence to bind in the hydrophobic groove of the vinculin head. While there are now many examples of proteins which are intrinsically disordered but which adopt a well-defined three-dimensional structure on binding to a protein or nucleic acid partner (e.g. Dyson and Wright 2005; Sugase et al. 2007), the observation of a protein which exists in a stable folded conformation but which must unfold in order to interact with a partner protein is much more unusual. Observations by a range of different biochemical and biophysical techniques have demonstrated this unusual “unfolding for binding” behaviour in VBS-containing constructs from different parts of the rod. These include:
-
The talin 482–655 five-helix bundle binds Vd1 only weakly. Removal of the C-terminal helix destabilises the helical bundle and leads to the partial unfolding of the protein, as shown by NMR, yet this is accompanied by tighter binding to vinculin (Papagrigoriou et al. 2004).
-
Talin 482–655 is stable to proteolysis by trypsin, whereas the four-helix construct talin 482–636 is readily degraded. In the complex of Vd1 with talin 482–636, Vd1 and the VBS1 sequence within the talin domain become stable to proteolysis, but all of the remaining talin helices are degraded (Papagrigoriou et al. 2004).
-
In the [1H,15N]-HSQC NMR spectrum of the complex between Vd1 and 15N-labeled talin 1843–1973, fewer cross-peaks are observed compared to the free protein, and these have a much smaller chemical shift dispersion. Cross-peaks which are not observed in the complex map onto helix H4 (the helix that binds vinculin), the C-terminal half of helix H3, and a short stretch at the C-terminus of helix H1. The highest cross-peak intensities correspond to helix H2 and the N-terminal part of helix H3 (Gingras et al. 2006). This provides direct evidence for substantial conformational mobility of those parts of the talin domain which are not in contact with vinculin, and hence for the unfolding of the helical bundle. Similar observations were made with talin 755–889 (Fillingham et al. 2005).
-
The talin 755–889 4-helix bundle has two threonine pairs (T775/T809 and T833/T867) within the hydrophobic core of the bundle (Fillingham et al. 2005). A mutant in which these were replaced by isoleucine/valine pairs (T775V/T809I/T833I/T867V) was much more stable than the wild-type construct but bound Vd1 much more weakly (Patel et al. 2006).
-
The effects of vinculin Vd1 binding on talin 1843–1973 have been studied by site-directed spin-labeling (Gingras et al. 2006). Cysteine residues were placed at key positions by mutagenesis; these were labeled with a nitroxide spin label to give singly or doubly spin-labeled proteins. Analysis of the EPR spectra to obtain information on the local mobility and on the distances between the nitroxides (Fig. 2) provided clear evidence that, on binding Vd1, the helical bundle unfolds as do at least two of the constituent helices, the VBS-containing helix 4 being immobilised in the complex.
The activation of vinculin binding to talin
The variations in the stability of the individual helical bundles that make up the talin rod are undoubtedly significant factors in determining vinculin binding. For the isolated domains, one could envisage either that the talin bundle is dynamic, and unfolds spontaneously to release the VBS which then binds to Vd1, or that an initial interaction of Vd1 with the folded talin bundle promotes unfolding of the latter. However, it is clear that the majority of the VBSs in intact talin are in a cryptic or low affinity state (Patel et al. 2006). Therefore, it appears that addition of vinculin is not in itself sufficient to lead to their activation.
Talin is required for the formation of the initial weak linkage between a fibronectin/integrin complex and actomyosin (Jiang et al. 2003), but reinforcement of this link by the recruitment of additional components is thought to be essential for focal adhesion assembly. Activation of the cryptic VBSs could increase the number of vinculin molecules bound simultaneously to talin and, because the vinculin tail binds F-actin (Ziegler et al. 2006), a progressive increase in vinculin binding to talin could provide a mechanism for a graduated strengthening of the link between integrins and the actin cytoskeleton. Since talin is clearly subject to the force exerted by actomyosin contraction and given that external mechanical force induces focal adhesion assembly (Galbraith et al. 2002), we have proposed that mechanical stretch may activate the VBSs in talin (Gingras et al. 2005; Papagrigoriou et al. 2004; Ziegler et al. 2006).
Direct evidence for this has recently come from elegant single molecule experiments on talin 482–889 reported by the Sheetz laboratory (del Rio et al. 2009). A construct containing residues 482–889 with a His-tag at the N-terminus and a biotin tag at the C-terminus was bound at one end to a Ni-NTA glass surface and at the other to an avidinated magnetic bead; the molecule could then be stretched by magnetic tweezers, and the binding of fluorescently labelled vinculin head detected by total internal reflection fluorescence (TIRF) microscopy. In the absence of mechanical force, binding of at most a single molecule of vinculin could be detected, which is consistent with earlier conclusions that many of the VBSs in this region are cryptic (Patel et al. 2006). However, the application of a force of 12pN to the talin led to a clear increase in the number of vinculin molecules bound, up to as many as three. Parallel force extension experiments showed mechanical unfolding of talin 482–889. These experiments demonstrate clearly that force-induced stretching can expose previously cryptic binding sites for vinculin in the N-terminal part of the talin rod (Fig. 3).
The pathway by which mechanical force unfolds talin bundles remains to be established, but recent steered molecular dynamics simulations on talin 482–889 (Hytonen and Vogel 2008) give an indication of the possibilities. The structure assumed for talin 482–889 was a five-helix bundle packed against a seven-helix bundle; this is a model (Fillingham et al. 2005) rather than an experimental structure. Two kinds of simulation were carried out: one in which the force was applied to the N– and C-termini of the molecule and one in which it was applied laterally in a distributed fashion along the length of the two terminal helices. In both cases, the structure broke apart during the simulation into three components, comprising helices 1–5, 6–8 and 9–12. However, when the force was applied to the termini, this was preceded by the unraveling of helices 1 and 12, followed by partial unraveling of helix 2, so that at least one of the VBSs (helix 12) would be in a random coil state and would have to re-form its helical conformation to bind vinculin. The different behaviour according to the direction of the applied force is interesting and may imply different unfolding and activation mechanisms for VBSs within domains in different parts of the rod. In the N-terminal region, where successive bundles pack closely against one another, the mechanical force is likely to act laterally on the helices, whereas in the C-terminal half of the rod, where the bundles have relatively flexible interfaces and end-to-end packing seems to be typical, the force would most probably act through the N– and C-termini of each bundle.
Conclusion
It is clear that a picture of talin in which it binds at one end to integrin and at the other to actin is too simplistic. Both the talin head and rod contain binding sites for integrins and F-actin, although the significance of this remains to be established. Both the head and the rod also bind acidic phospholipids, and it will be important to establish whether the molecule lies along the cytoplasmic face of the membrane. It is also clear that talin exists in several conformational states (monomer/dimer and open and closed states). Much of talin exists in the cytoplasm in an autoinhibited form in which the talin rod masks the integrin binding site in the talin head, and the relative importance of the Rap1/RIAM and PIPKIγ90/PIP2 pathways in talin activation remain to be explored. Moreover, it has now been established from in vitro studies that the conformation of the talin rod is regulated by force, and it will be necessary to develop methods that allow this and the recruitment of vinculin to integrin/talin/actin complexes to be explored in real time and in a cellular context.
References
Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW, Liddington RC (2004) Structural basis for vinculin activation at sites of cell adhesion. Nature 430:583–586. doi:10.1038/nature02610
Barsukov IL, Prescot A, Bate N, Patel B, Floyd DN, Bhanji N, Bagshaw CR, Letinic K, Di Paolo G, De Camilli P, Roberts GCK, Critchley DR (2003) Phosphatidylinositol phosphate kinase type 1γ and β1-integrin cytoplasmic domain bind to the same region in the talin FERM domain. J Biol Chem 278:31202–31209. doi:10.1074/jbc.M303850200
Bass MD, Smith BJ, Prigent SA, Critchley DR (1999) Talin contains three similar vinculin-binding sites predicted to form an amphipathic helix. Biochem J 341:257–263. doi:10.1042/0264-6021:3410257
Bass MD, Patel B, Barsukov IG, Fillingham IJ, Mason R, Smith BJ, Bagshaw CR, Critchley DR (2002) Further characterization of the interaction between the cytoskeletal proteins talin and vinculin. Biochem J 362:761–768. doi:10.1042/0264-6021:3620761
Borgon RA, Vonrhein C, Bricogne G, Bois PR, Izard T (2004) Crystal structure of human vinculin. Structure 12:1189–1197. doi:10.1016/j.str.2004.05.009
Borowsky ML, Hynes RO (1998) Layilin, a novel talin-binding transmembrane protein homologous with C-type lectins, is localized in membrane ruffles. J Cell Biol 143:429–442. doi:10.1083/jcb.143.2.429
Bouaouina M, Lad Y, Calderwood DA (2008) The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J Biol Chem 283:6118–6125. doi:10.1074/jbc.M709527200
Brett TJ, Legendre-Guillemin V, McPherson PS, Fremont DH (2006) Structural definition of the F-actin-binding THATCH domain from HIP1R. Nat Struct Mol Biol 13:121–130. doi:10.1038/nsmb1043
Calderwood DA (2004) Integrin activation. J Cell Sci 117:657–666. doi:10.1242/jcs.01014
Calderwood DA, Yan BX, de Pereda JM, Alvarez BG, Fujioka Y, Liddington RC, Ginsberg MH (2002) The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem 277:21749–21758. doi:10.1074/jbc.M111996200
Campbell ID, Ginsberg MH (2004) The talin-tail interaction places integrin activation on FERM ground. Trends Biochem Sci 29:429–435. doi:10.1016/j.tibs.2004.06.005
Cheung TYS, Fairchild MJ, Zarivach R, Tanentzapf G, van Petegem F (2009) Crystal structure of the talin integrin binding domain 2. J Mol Biol 387:787–793. doi:10.1016/j.jmb.2009.01.053
Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B (2005) The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J Cell Biol 171:383–392. doi:10.1083/jcb.200503017
Cohen DM, Chen H, Johnson RP, Choudhury B, Craig SW (2005) Two distinct head-tail interfaces cooperate to suppress activation of vinculin by talin. J Biol Chem 280:17109–17117. doi:10.1074/jbc.M414704200
Critchley DR (2009) Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys 38:235–254. doi:10.1146/annurev.biophys.050708.133744
Critchley DR, Gingras AR (2008) Talin at a glance. J Cell Sci 121:1345–1347. doi:10.1242/jcs.018085
de Pereda JM, Wegener KL, Santelli E, Bate N, Ginsberg MH, Critchley DR, Campbell ID, Liddington RC (2005) Structural basis for phosphatidylinositol phosphate kinase type Iγ binding to talin at focal adhesions. J Biol Chem 280:8381–8386. doi:10.1074/jbc.M413180200
del Rio A, Perez-Jimenez R, Liu RC, Roca-Cusachs P, Fernandez JM, Sheetz MP (2009) Stretching single talin rod molecules activates vinculin binding. Science 323:638–641. doi:10.1126/science.1162912
Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P (2002) Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature 420:85–89. doi:10.1038/nature01147
Dietrich C, Goldmann WH, Sackmann E, Isenberg G (1993) Interaction of NBD-talin with lipid monolayers—a film balance study. FEBS Lett 324:37–40. doi:10.1016/0014-5793(93) 81527-7
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208. doi:10.1038/nrm1589
Fillingham I, Gingras AR, Papagrigoriou E, Patel B, Emsley J, Critchley DR, Roberts GC, Barsukov IL (2005) A vinculin binding domain from the talin rod unfolds to form a complex with the vinculin head. Structure 13:65–74. doi:10.1016/j.str.2004.11.006
Galbraith CG, Yamada KM, Sheetz MP (2002) The relationship between force and focal complex development. J Cell Biol 159:695–705. doi:10.1083/jcb.200204153
Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC (2003) Structural determinants of integrin recognition by talin. Mol Cell 11:49–58. doi:10.1016/S1097-2765(02) 00823-7
Gilmore AP, Burridge K (1996) Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4, 5-bisphosphate. Nature 381:531–535. doi:10.1038/381531a0
Gingras AR, Ziegler WH, Frank R, Barsukov IL, Roberts GCK, Critchley DR, Emsley J (2005) Mapping and consensus sequence identification for multiple vinculin binding sites within the talin rod. J Biol Chem 280:37217–37224. doi:10.1074/jbc.M508060200
Gingras AR, Vogel KP, Steinhoff HJ, Ziegler WH, Patel B, Emsley J, Critchley DR, Roberts GCK, Barsukov IL (2006) Structural and dynamic characterization of a vinculin binding site in the talin rod. Biochemistry 45:1805–1817. doi:10.1021/bi052136l
Gingras AR, Bate N, Goult BT, Hazelwood L, Canestrelli I, Grossmann JG, Liu H, Putz NSM, Roberts GCK, Volkmann N, Hanein D, Barsukov IL, Critchley DR (2008) The structure of the C-terminal actin-binding domain of talin. EMBO J 27:458–469. doi:10.1038/sj.emboj.7601965
Gingras AR, Ziegler WH, Bobkov AA, Joyce MG, Fasci D, Himmel M, Rothemund S, Ritter A, Grossmann JG, Patel B, Bate N, Goult BT, Emsley J, Barsukov IL, Roberts GCK, Liddington RC, Ginsberg MH, Critchley DR (2009) Structural determinants of integrin-binding to the talin rod. J Biol Chem (in press). doi:10.1074/jbc.M805937200
Goksoy E, Ma YQ, Wang XX, Kong XM, Perera D, Plow EF, Qin J (2008) Structural basis for the autoinhibition of talin in regulating integrin activation. Mol Cell 31:124–133. doi:10.1016/j.molcel.2008.06.011
Goldmann WH, Senger R, Kaufmann S, Isenberg G (1995) Determination of the affinity of talin and vinculin to charged lipid vesicles: a light scattering study. FEBS Lett 368:516–518. doi:10.1016/0014-5793(95) 00678-3
Goult BT, Bate N, Anthis NJ, Wegener KL, Gingras AR, Patel B, Barsukov IL, Campbell ID, Roberts GCK, Critchley DR (2009) The structure of an interdomain complex which regulates talin activity. J Biol Chem (in press). doi:10.1074/jbc.M900078200
Han JW, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH (2006) Reconstructing and deconstructing agonist-induced activation of integrin αIIbß3. Curr Biol 16:1796–1806. doi:10.1016/j.cub.2006.08.035
Harburger DS, Calderwood DA (2009) Integrin signalling at a glance. J Cell Sci 122:159–163. doi:10.1242/jcs.018093
Hemmings L, Rees DJG, Ohanian V, Bolton SJ, Gilmore AP, Patel B, Priddle H, Trevithick JE, Hynes RO, Critchley DR (1996) Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J Cell Sci 109:2715–2726
Hynes RO (2002) Integrins: Bidirectional, allosteric signaling machines. Cell 110:673–687. doi:10.1016/S0092-8674(02)00971-6
Hytonen VP, Vogel V (2008) How force might activate talin’s vinculin binding sites: SMD reveals a structural mechanism. PLOS Comput Biol 4. doi:10.1371/journal.pcbi.0040024
Izard T, Evans G, Borgon RA, Rush CL, Bricogne G, Bois PRJ (2004) Vinculin activation by talin through helical bundle conversion. Nature 427:171–175. doi:10.1038/nature02281
Jiang GY, Giannone G, Critchley DR, Fukumoto E, Sheetz MP (2003) Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424:334–337. doi:10.1038/nature01805
Johnson RP, Craig SW (1994) An intramolecular association between the head and tail domains of vinculin modulates talin binding. J Biol Chem 269:12611–12619
Johnson RP, Craig SW (1995a) The carboxy-terminal tail domain of vinculin contains a cryptic binding site for acidic phospholipids. Biochem Biophys Res Commun 210:159–164. doi:10.1006/bbrc.1995.1641
Johnson RP, Craig SW (1995b) F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature 373:261–264. doi:10.1038/373261a0
Larjava H, Plow EF, Wu C (2008) Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep 9:1203–1208. doi:10.1038/embor.2008.202
Lee HS, Bellin RM, Walker DL, Patel B, Powers P, Liu HJ, Garcia-Alvarez B, de Pereda JM, Liddington RC, Volkmann N, Hanein D, Critchley DR, Robson RM (2004) Characterization of an actin-binding site within the talin FERM domain. J Mol Biol 343:771–784. doi:10.1016/j.jmb.2004.08.069
Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH (2009) RIAM activates integrins by linking talin to RAS GTPase membrane-targeting sequences. J Biol Chem 284:5119–5127. doi:10.1074/jbc.M807117200
Legate KR, Fassler R (2009) Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci 122:187–198. doi:10.1242/jcs.041624
Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA (2002) Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420:89–93. doi:10.1038/nature01082
Ling K, Doughman RL, Iyer VV, Firestone AJ, Bairstow SF, Mosher DF, Schaller MD, Anderson RA (2003) Tyrosine phosphorylation of type Iγ phosphatidylinositol phosphate kinase by src regulates an integrin-talin switch. J Cell Biol 163:1339–1349. doi:10.1083/jcb.200310067
Martel V, Racaud-Sultan C, Dupe S, Marie C, Paulhe F, Galmiche A, Block MR, Albiges-Rizo C (2001) Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J Biol Chem 276:21217–21227. doi:10.1074/jbc.M102373200
McCann RO, Craig SW (1997) The I/LWEQ module: A conserved sequence that signifies F-actin binding in functionally diverse proteins from yeast to mammals. Proc Natl Acad Sci USA 94:5679–5684. doi:10.1073/pnas.94.11.5679
Moes M, Rodius S, Coleman SJ, Monkley SJ, Goormaghtigh E, Tremuth L, Kox C, van der Holst PPG, Critchley DR, Kieffer N (2007) The integrin binding site 2 (IBS2) in the talin rod domain is essential for linking integrin β subunits to the cytoskeleton. J Biol Chem 282:17280–17288. doi:10.1074/jbc.M611846200
Molony L, McCaslin D, Abernethy J, Paschal B, Burridge K (1987) Properties of talin from chicken gizzard smooth muscle. J Biol Chem 262:7790–7795
Monkley SJ, Pritchard CA, Critchley DR (2001) Analysis of the mammalian talin2 gene TLN2. Biochem Biophys Res Commun 286:880–885. doi:10.1006/bbrc.2001.5497
Niggli V, Kaufmann S, Goldmann WH, Weber T, Isenberg G (1994) Identification of functional domains in the cytoskeletal protein talin. Eur J Biochem 224:951–957. doi:10.1111/j.1432-1033.1994.00951.x
Papagrigoriou E, Gingras AR, Barsukov IL, Bate N, Fillingham IJ, Patel B, Frank R, Ziegler WH, Roberts GCK, Critchley DR, Emsley J (2004) Activation of a vinculin-binding site in the talin rod involves rearrangement of a five-helix bundle. EMBO J 23:2942–2951. doi:10.1038/sj.emboj.7600285
Patel B, Gingras AR, Bobkov AA, Fujimoto LM, Zhang M, Liddington RC, Mazzeo D, Emsley J, Roberts GCK, Barsukov IL, Critchley DR (2006) The activity of the vinculin binding sites in talin is influenced by the stability of the helical bundles that make up the talin rod. J Biol Chem 281:7458–7467. doi:10.1074/jbc.M508058200
Rodius S, Chaloin O, Moes M, Schaffner-Reckinger E, Landrieu I, Lippens G, Lin MH, Zhang J, Kieffer N (2008) The talin rod IBS2 α-helix interacts with the β3 integrin cytoplasmic tail membrane-proximal helix by establishing charge complementary salt bridges. J Biol Chem 283:24212–24223. doi:10.1074/jbc.M709704200
Senetar MA, Foster SJ, McCann RO (2004) Intrasteric inhibition mediates the interaction of the I/LWEQ module proteins talin1, talin2, Hip1, and Hip12 with actin. Biochemistry 43:15418–15428. doi:10.1021/bi0487239
Senetar MA, McCann RO (2005) Gene duplication and functional divergence during evolution of the cytoskeletal linker protein talin. Gene 362:141–152. doi:10.1016/j.gene.2005.08.012
Smith SJ, McCann R (2007) A C-terminal dimerization motif is required for focal adhesion targeting of talin 1 and the interaction of the talin 1 I/LWEQ module with F-actin. Biochemistry 46:10886–10898. doi:10.1021/bi700637a
Sugase K, Dyson HJ, Wright PE (2007) Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447:1021–1023. doi:10.1038/nature05858
Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA (2003) Talin binding to integrin beta tails: A final common step in integrin activation. Science 302:103–106. doi:10.1126/science.1086652
Tremuth L, Kreis S, Melchior C, Hoebeke J, Ronde P, Plancon S, Takeda K, Kieffer N (2004) A fluorescence cell biology approach to map the second integrin-binding site of talin to a 130-amino acid sequence within the rod domain. J Biol Chem 279:22258–22266. doi:10.1074/jbc.M400947200
Watanabe N, Bodin L, Pandey M, Krause M, Coughlin S, Boussiotis VA, Ginsberg MH, Shattil SJ (2008) Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin αIIbß3. J Cell Biol 181:1211–1222. doi:10.1083/jcb.200803094
Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID (2007) Structural basis of integrin activation by talin. Cell 128:171–182. doi:10.1016/j.cell.2006.10.048
Wegener KL, Basran J, Bagshaw CR, Campbell ID, Roberts GCK, Critchley DR, Barsukov IL (2008) Structural basis for the interaction between the cytoplasmic domain of the hyaluronate receptor layilin and the talin F3 subdomain. J Mol Biol 382:112–126. doi:10.1016/j.jmb.2008.06.087
Winkler J, Lunsdorf H, Jockusch BM (1997) Energy-filtered electron microscopy reveals that talin is a highly flexible protein composed of a series of globular domains. Eur J Biochem 243:430–436. doi:10.1111/j.1432-1033.1997.0430a.x
Xing BD, Jedsadayanmata A, Lam SCT (2001) Localization of an integrin binding site to the C terminus of talin. J Biol Chem 276:44373–44378. doi:10.1074/jbc.M108587200
Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9:858–867. doi:10.1038/ncb0807-858
Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP (2008) Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol 10:1062–1068. doi:10.1038/ncb1765
Ziegler WH, Liddington RC, Critchley DR (2006) The structure and regulation of vinculin. Trends Cell Biol 16:453–460. doi:10.1016/j.tcb.2006.07.004
Acknowledgements
We are most grateful to all of our colleagues and collaborators whose names appear in the references for their indispensable contributions to the work in our laboratory. We also thank Alex Gingras for his assistance with the figures. The work in our laboratory is currently supported by grants from the Wellcome Trust, Cancer Research UK and the NIH Cell Migration Consortium (Grant U54 GM64346 from the National Institute of General Medical Sciences).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
An annotated reference list is available as Electronic Supplementary Material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Roberts, G.C.K., Critchley, D.R. Structural and biophysical properties of the integrin-associated cytoskeletal protein talin. Biophys Rev 1, 61–69 (2009). https://doi.org/10.1007/s12551-009-0009-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-009-0009-4