Abstract
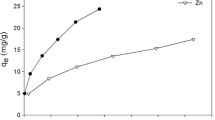
The most appropriate method in designing the adsorption systems and assessing the performance of the adsorption systems is to have an idea on adsorption isotherms. Comparison analysis of linear least square method and nonlinear method for estimating the isotherm parameters was made using the experimental equilibrium data of Zn(II) and Cu(II) onto kaolinite. Equilibrium data were fitted to Freundlich, Langmuir, and Redlich–Peterson isotherm equations. In order to confirm the best-fit isotherms for the adsorption system, the data set using the chi-square (χ 2), combined with the values of the determined coefficient (r 2) was analyzed. Nonlinear method was found to be a more appropriate method for estimating the isotherm parameters. The best fitting isotherm was the Langmuir and Redlich–Peterson isotherm. The Redlich–Peterson is a special case of Langmuir when the Redlich–Peterson isotherm constant g was unity. The sorption capacity of kaolinite to uptake metal ions in the increasing order was given by Cu (4.2721 mg/g) < Zn (4.6710 mg/g).



Similar content being viewed by others
References
Al-Jlil SA, Alsewailem FD (2009) Saudi Arabian clays for lead removal in wastewater. Appl Clay Sci 42(3–4):671–674
Al-Othman ZA, Habila MA, Hashem A (2012) Removal of zinc(II) from aqueous solutions using modified agricultural wastes: kinetics and equilibrium studies. Arab J Geosci 5:1–11
Alvarez-Ayuso E, Garica-Sanchez A (2003) Removal of heavy metals from waste waters by natural and Na-exchanged bentonites. Clays Clay Miner 51(5):475–480
Ayoub GM, Semerjian L, Acra A, El Fadel M, Koopman B (2001) Heavy metal removal by coagulation with seawater liquid bittern. J Environ Eng 127(3):196–202
Bhattacharya KG, Gupta SS (2008) Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl Clay Sci 41(1–2):1–9
Bhattacharya KG, Mandal SN, Das SK (2006) Adsorption of Zn(II) from aqueous solution by using different adsorbents. Chem Eng J 123(1–2):43–51
Bose P, Bose MA, Kumar S (2002) Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc, and cyanide. Adv Environ Res 7(1):179–195
Ghafari B, Khezri SM (2013) Evaluation of hydrophilic cotton processing wastewater treatment methods and giving the optimum method for it. Arab J Geosci 6(8):2991–2995
Gosset T, Trancart JL, Thévenot DR (1986) Batch metal removal by peat: kinetics and thermodynamics. Water Res 20(1):21–26
Ho YS (2004) Selection of optimum sorption isotherm. Carbon 42(10):2115–2117
Ho YS (2006) Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods. Pol J Environ Stud 15(1):81–86
Ho YS, Chiu WT, Wang CC (2005) Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Bioresource Technol 96(11):1285–1291
Hussain S, Gul S, Khan S, Rehman H (2012) Retention studies of chromium(VI) from aqueous solution on the surface of a novel carbonaceous material. Arab J Geosci 5(6):1–10
Ibrahim KM, Khoury H (2002) Use of natural chabazite–phillipsite tuff in wastewater treatment from electroplating factories in Jordan. Environ Geol 41(5):547–551
Jiang MQ, Jin XY, Lu XQ, Chen ZL (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252(1):33–39
Jokela P, Keskitalo P (1999) Plywood mill water system closure by dissolved air flotation treatment. Water Sci Tech 40(11–12):33–42
Khan S, Ishaq M, Ahmad I, Hussain S, Ullah H (2013) Evaluation of coal as adsorbent for phosphate removal. Arab J Geosci 6(4):1113–1117
Khodaverdiloo H, Samadi A, Motlagh MB, Dashtaki SG, Han F (2013) Characterization of parameters of sorption isotherm models of cadmium in selected semi-arid region agricultural cambisols and calcisols. Arab J Geosci 6(9):3389–3395
Kumar KV (2006) Comparative analysis of linear and non-linear method of estimating the sorption isotherm parameters for malachite green onto activated carbon. J Hazard Mater 136(2):197–202
Kumar KV, Sivanesan S (2006) Isotherm parameters for basic dyes onto activated carbon: comparison of linear and non-linear method. J Hazard Mater 129(1–3):147–150
Kumar KV, Sivanesan S (2007) Sorption isotherm for safranin onto rice husk: comparison of linear and non-linear methods. Dyes Pigments 72(1):130–133
Matis KA, Zouboulis AI, Lazaridis NK, Hancock IC (2003) Sorptive flotation for metal ions recovery. Int J of Miner Process 70(1–4):99–108
Ouki SK, Kavanagh M (1997) Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Manage Res 15(4):383–394
Panday KK, Prased G, Singh VN (1985) Copper(II) removal from aqueous solution by fly ash. Water Res 19(7):869–873
Perić J, Trago M, Vukojević Medvidović N (2004) Removal of zinc, copper and lead by natural zeolite—a comparison of adsorption isotherms. Water Res 38(7):1893–1899
Potgieter JH, Vermaak SS, Kalibantonga PD (2006) Heavy metals removal from solution by palygorskite clay. Miner Eng 19(5):463–470
Richter E, Schutz W, Myers AL (1989) Effect of adsorption equation on prediction of multicomponent adsorption equilibria by the ideal adsorbed solution theory. Chem Eng Sci 44(8):1609–1616
Sari A, Tuzen M (2008) Removal of Cr(VI) from aqueous solution by Turkish vermiculite: equilibrium, thermodynamic and kinetic studies. Sep Sci Technol 43(13):3563–3581
Sari A, Tuzen M (2009) Kinetic and equilibrium studies of Pb(II) and Cd(II) removal from aqueous solution onto colemanite ore waste. Desalination 249(1):260–266
Sari A, Tuzen M, Çitak D, Soylak M (2007a) Adsorption characteristics of Cu(II) and Pb(II) onto expanded perlite from aqueous solution. J Hazard Mater 148(1–2):387–394
Sari A, Tuzen M, Soylak M (2007b) Adsorption of Pb(II) and Cr(III) from aqueous solution on Celtek clay. J Hazard Mater 144(1–2):41–46
Sari A, Çitak D, Tuzen M (2010) Equilibrium, thermodynamic and kinetic studies on adsorption of Sb(III) from aqueous solution using low-cost natural diatomite. Chem Eng J 162(2):521–527
Semerjian L, Ayoub GM (2003) High-pH-magnesium coagulation–flocculationin wastewater treatment. Adv Environ Res 7(2):389–403
Shahmohammadi-Kalalagh S, Babazadeh H (2013) Isotherms for the sorption of zinc and copper onto kaolinite: comparison of various error functions. Int J Environ Sci Technol. doi:10.1007/s13762-013-0260-x
Shahmohammadi-Kalalagh S, Babazadeh H, Nazemi AH, Manshouri M (2011) Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Caspian J Env Sci 9(2):243–255
Singh AK, Singh DP, Singh VN (1998) Removal of Zn(II) from water by adsorption on China clay. Environ Technol Lett 9(10):1153–1162
Sposito G (1989) The chemistry of soils. Oxford University Press, New York
Subramanyam B, Das A (2009) Linearized and non-linearized isotherm models comparative study on adsorption of aqueous phenol solution in soil. Int J Environ Sci Tech 6(4):633–640
Undaybeytia López T, Morillo González E, Maqueda Porras C (1996) Adsorption of Cd and Zn on montmorillonite in the presence of a cationic pesticide. Clay Miner 31(4):485–490
Vimonses V, Lei S, Jin B, Chow WK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem Eng J 148(2–3):354–364
Wingenfelder U, Hansen C, Furrer G, Schulin R (2005) Removal of heavy metals from mine water by natural zeolites. Environ Sci Technol 39(2):4606–4613
Yang XJ, Fane AG, MacNaughton S (2001) Removal and recovery of heavy metals from wastewatr by supported liquid membranes. Water Sci Technol 43(2):341–348
Yong RN, Mohamed AMO, Warketin BP (1992) Principles of contaminants transport in soil. Elsevier, Amsterdam
Zhu S, Hou H, Xue Y (2008) Kinetic and isothermal studies of lead ion adsorption onto bentonite. Appl Clay Sci 40(1–4):171–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahmohammadi-Kalalagh, S., Babazadeh, H. & Nazemi, A. Comparison of linear and nonlinear forms of isotherm models for Zn(II) and Cu(II) sorption on a kaolinite. Arab J Geosci 8, 397–402 (2015). https://doi.org/10.1007/s12517-013-1114-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-013-1114-z