Abstract
Introduction
Type-2 diabetes mellitus (T2DM) is a progressive disease, and many patients eventually require insulin therapy. This study examined real-world outcomes of switching basal insulin analogs among patients with T2DM.
Methods
Using two large United States administrative claims databases (IMPACT® and Humana®), this longitudinal retrospective study examined two cohorts of adult patients with T2DM. Previously on insulin glargine, Cohort 1 either continued insulin glargine (GLA-C) or switched to insulin detemir (DET-S), while Cohort 2 was previously on insulin detemir, and either continued insulin detemir (DET-C) or switched to insulin glargine (GLA-S). One-year follow-up treatment persistence and adherence, glycated hemoglobin (HbA1c), hypoglycemia events, healthcare utilization and costs were assessed. Selection bias was minimized by propensity score matching between treatment groups within each cohort.
Results
A total of 5,921 patients (mean age 60 years, female 50.0%, HbA1c 8.6%) were included in the analysis (Cohort 1: IMPACT®: n = 536 DET-S matched to n = 2,668 GLA-C; Humana®: n = 256 DET-S matched to n = 1,262 GLA-C; Cohort 2: n = 419 GLA-S matched to n = 780 DET-C), with similar baseline characteristics between treatment groups in each cohort. During 1-year follow-up, in Cohort 1, DET-S patients, when compared with GLA-C patients, had lower treatment persistence/adherence with 33–40% restarting insulin glargine, higher rapid-acting insulin use, worse HbA1c outcomes, significantly higher diabetes drug costs, and similar hypoglycemia rates, health care utilization and total costs. However, in Cohort 2 overall opposite outcomes were observed and only 19.8% GLA-S patients restarted insulin detemir.
Conclusions
This study showed contrasting clinical and economic outcomes when patients with T2DM switched basal insulin analogs, with worse outcomes observed for patients switching from insulin glargine to insulin detemir and improved outcomes when switching from insulin detemir to insulin glargine. Further investigation into the therapeutic interchangeability of insulin glargine and insulin detemir in the real-world setting is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guidelines from the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) note that most patients with long-standing type 2 diabetes mellitus (T2DM) will need insulin at some point during their treatment [1]. Although the long-acting insulin analogs glargine and detemir improve glycemic control with a low risk of hypoglycemia in patients with T2DM [2], initiation of insulin treatment is often delayed [3].
Many studies have been conducted to examine the initiation of insulin glargine and insulin detemir among T2DM patients. A 52-week, open-label study compared outcomes in patients with T2DM treated with insulin glargine or insulin detemir as part of a basal-bolus regimen (n = 319) [4]. Both insulin glargine and insulin detemir improved glycemic control, with similar overall effects in blood sugar control and low incidence of hypoglycemia for both. A Cochrane Database Review comparing insulin glargine and insulin detemir in randomized clinical trials likewise concluded that there were no differences in glycemic control between these two insulins, although twice-daily dosing and higher doses were more often needed with insulin detemir than with insulin glargine [5]. In addition, the “Effect of Insulin Detemir and Insulin Glargine on Blood Glucose Control in Subjects With Type 2 Diabetes” trial (EFFICACY: NCT00909480)—investigating the once-daily dosing of basal insulin as add-on to metformin over 26 weeks—showed that, while both improved glycemic control when added to metformin, the use of insulin glargine resulted in greater reductions in glycated hemoglobin (HbA1c) as compared with insulin detemir [6].
In addition to these clinical studies, a few studies have also been conducted to examine the effects of these long-acting insulin analogs in a real-world setting. These real-world studies have reported somewhat conflicting data. Xie et al., for example, reported that patients initiating insulin glargine were more likely to persist with and adhere to treatment, and to show better glycemic control and similar overall hypoglycemia rate with no increase in healthcare costs [7] or weight change [8], compared with those initiating insulin detemir. In contrast, a study of 306 patients enrolled in a large United States (US) managed care organization found that glycemic control after 180 days was similar for insulin glargine and insulin detemir [9]. In addition, McAdam-Marx et al. reported that patients with T2DM initiating insulin detemir were 30% less likely to gain 0.9 kg or more in body weight, with no significant difference in HbA1c values, compared with insulin glargine [10].
Although there is much evidence available on the initiation of basal analog insulins, very few studies have investigated the effects of switching from one basal insulin to another. Where studies have been conducted, their results have been conflicting. In a randomized double-blind crossover study in patients with T2DM, King et al. reported that once-daily dosing of insulin detemir and insulin glargine provided similar 24-h glycemic control [11]. However, this was a study of short duration and with only 36 patients. The “Predictable Results and Experience in Diabetes through Intensification and Control to Target: an International Variability Evaluation” (PREDICTIVE: NCT00659295) study was an observational study designed to evaluate switching from insulin glargine to insulin detemir (primarily with respect to adverse events) in patients with type 1 or T2DM [12]. In a European cohort of patients (n = 777) in the PREDICTIVE study, there were significant improvements in HbA1c with fewer hypoglycemic events after switching to insulin detemir [13]. Using the framework of cost-effectiveness analysis, an Asian study based on data from a Korean cohort of the PREDICTIVE study reported reduced total diabetes care costs and increased life expectancy of 0.06 years after switching from insulin glargine to insulin detemir [14]. A small retrospective study recently evaluated outcomes in patients (n = 10 with type 1 diabetes and n = 21 with T2DM) switching from insulin glargine to insulin detemir due to changes in Medicaid formulary coverage [15]. Among patients with T2DM, both insulin dose and the proportion of patients needing twice-daily dosing were significantly higher after switching to insulin detemir. Despite 33% higher daily dosing after switching to insulin detemir, HbA1c levels were not improved compared with insulin glargine. More recently, the “Retrospective Evaluation of a Long-acting Insulin Switch on Hemoglobin HbA1c” (RELISH) study found that HbA1c levels increased significantly after patients converted from insulin glargine to insulin detemir, with no change in the proportion of patients achieving a goal of HbA1c < 7.0% [16]. Furthermore, 22% of patients (9/41) switched from insulin detemir back to insulin glargine during the RELISH study. No published study examined switching from insulin detemir to insulin glargine.
Designed to expand upon these earlier reports, the current study investigated real-world outcomes among patients with T2DM switching basal insulin analogs, either from insulin glargine to insulin detemir or from insulin detemir to insulin glargine, by evaluating two large independent national US databases consisting of commercially insured and Medicare populations.
Methods
This study was a retrospective longitudinal cohort study, combining data from two large, independent US national healthcare administrative databases, consisting of commercially insured (IMPACT®, Waltham, USA) and Medicare (Humana, Louisville, USA) populations from 2006 to 2012. The Ingenix IMPACT® National Managed Care Benchmark Database comprises about 50 US healthcare plans and contains medical claims, pharmacy claims, eligibility data, and laboratory results for 107 million patients, of whom 73% had pharmacy benefits and 18% had laboratory results. Humana’s administrative claims database contains medical, pharmacy, and laboratory claims for over 12 million members of Medicare, commercial, and Medicaid health plans. It includes claims information from more than five million Medicare members through Medicare Advantage plans, as well as prescription drug coverage data from throughout the US.
Patients
Patients diagnosed with T2DM, defined as having ≥1 inpatient visit or ≥2 physician visits (≥30 days apart) with a primary or secondary diagnosis of T2DM [International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes: 250.x0 or 250.x2] [17] were included in the current analysis. Patients were further required to be ≥18 years of age, to have had continuous pharmacy and medical benefit coverage for ≥6 months before the index date (baseline) and for ≥12 months after the index date (follow-up), and to have had ≥1 HbA1c test result obtained during the baseline period (within 6-month period before the index date until 15 days after the index date).
Two cohorts were identified for the analyses in this study. Cohort 1 included patients who were treated with insulin glargine and subsequently either switched to insulin detemir (DET-S) or remained on insulin glargine (GLA-C). For the DET-S group, the index date is the initial switching date to detemir. The GLA-C group consisted of patients with ≥3 prescription drug claims for insulin glargine and no claims for insulin detemir, and their index date was chosen as a randomly selected date between the third and last insulin glargine claims dates. For both groups, patients were required to have at least one pharmacy claim for insulin glargine in each quarter during the baseline period, but not for premix or other basal insulin. Similarly, Cohort 2 included patients treated with insulin detemir and who subsequently either switched to insulin glargine (GLA-S) or remained on insulin detemir (DET-C). For the GLA-S group, the index date is the initial switching date to glargine. The DET-C group consisted of patients with ≥3 prescription drug claims for insulin detemir and no claims for insulin glargine, and their index date was chosen as a randomly selected date between the third and last insulin detemir claims dates. For both groups, patients were required to have at least one insulin detemir claim with each quarter, but no pharmacy claims for premix or other basal insulin during the baseline period.
Assessments
Baseline demographic and clinical characteristics were examined for all patients, using data recorded within 6 months before the index date. These variables included age at the index date, gender, region, HbA1c level, oral anti-diabetes drugs (OAD) and rapid/regular insulin use, comorbidities, hypoglycemia rates, types of insurance coverage, copay of the index drug, device type (vial vs pen) of the baseline insulin therapy and of the index insulin therapy, and total and diabetes-related healthcare utilization and costs. Baseline HbA1c data were from HbA1c test level dated between 6 months before the index date and 15 days after. If patients had multiple HbA1c results during this period, the value dated closest to the index date was used as the baseline value. Outcome comparisons between GLA-C and DET-S in Cohort 1 and between GLA-S and DET-C in Cohort 2 were made over 1 year of follow-up and consisted of insulin use, treatment persistence and adherence, clinical endpoints, hypoglycemic events, and healthcare resource utilization.
Insulin use endpoints included treatment persistence and adherence, daily average consumption (DACON) of insulin glargine or insulin detemir, and utilization of rapid-acting insulin (RAI). Treatment persistence was defined as patients remaining on the index insulin (insulin glargine for GLA-C and GLA-S, and insulin detemir for DET-C and DET-S) during the follow-up period without discontinuation after the index date [7, 8, 18–20]. Study medication was considered discontinued if the prescription was not refilled within the expected time of medication coverage (the 90th percentile of the time, stratified by the metric quantity supplied, between first and second fills among patients with at least one refill). Patients who restarted their initial medication after a period without it during follow-up were considered non-persistent. Persistence days were measured as the number of days between index date and discontinuation date. Sensitivity analyses were also conducted using 75th and 95th percentiles of the time.
Treatment adherence was measured by both the traditional medication possession ratio (MPR) and the adjusted MPR, which takes into account the differences in insulin device package sizes [21], used as both continuous and dichotomized variables (adherent, MPR ≥0.8; non-adherent, MPR <0.8). The adjusted MPR was calculated by multiplying the traditional MPR (the total days’ supply of all filled study drug prescriptions in the analysis period divided by the number of days in the analysis period) by the average days between prescription refills for patients using insulin divided by the average days’ supply for patients using insulin. The DACON was calculated as the total number of units dispensed before the last refill of study drug divided by the total number of days between initiation and last refill during follow-up. During the follow-up, the percentages of patients from the DET-S group restarting insulin glargine and from the GLA-S group restarting insulin detemir were also identified.
Clinical endpoints included HbA1c levels and hypoglycemic events. The HbA1c assessments included the change in HbA1c from baseline values and the percentage of patients achieving pre-specified targets of HbA1c < 7.0% and <8.0% during the 1 year of follow-up: while an HbA1c target of <7.0% is recommended for most patients with T2DM, less stringent goals are recommended for some patients, including those with limited life expectancy, marked comorbidity, or history of severe hypoglycemia [22]. As such, the National Committee for Quality Assurance Diabetes Recognition Program includes both HbA1c levels as measures related to glycemic control [23].
Hypoglycemic events were identified and counted using medical claims during the baseline and follow-up periods. A hypoglycemic event was identified via ICD-9-CM diagnosis codes in any position, based on the algorithm published by Ginde et al. [24]. In addition, hypoglycemic events occurring at specific settings [inpatient and/or emergency department (ED) or ambulatory (outpatient visit or physician office visit)] were identified and the setting used as a proxy for severity of hypoglycemia. The number of hypoglycemic events overall and events by setting was counted (one per unique setting per day); both the proportion of patients with at least one hypoglycemia event (prevalence rate) and the average number of hypoglycemia events per patient (event rate) during the 1-year follow-up period were identified.
Healthcare resource utilization included outpatient visits, ED visits, inpatient admissions, inpatient length of stay (days), and endocrinologist visits. Diabetes-related healthcare resource utilization included claims with a primary or secondary diagnosis of diabetes (ICD-9-CM: 250.xx). Healthcare costs were computed as total paid amounts of adjudicated claims (sum of plan-paid amounts, patients’ out-of-pocket payments, and third party payments). Total all-cause healthcare costs included inpatient, ED, outpatient, and prescription drug costs, and diabetes-related healthcare costs included costs from medical claims for inpatient, ED, or outpatient visits, with a primary or secondary diagnosis of diabetes (ICD-9-CM: 250.xx), anti-diabetes medications, and glucose meters and test strips. All costs were adjusted to 2011 levels.
Data Analysis
To address potential selection bias in the real-world setting, patients in both cohorts were matched by propensity score matching (PSM) to balance baseline demographic, clinical, and economic characteristics. The variables included in the PSM model were age, gender, copay, health plan, geographic region, initial year, baseline comorbidities, anti-diabetes drug, concomitant medications, baseline HbA1c categories, all-cause and diabetes-related utilizations and costs, diabetes education and initiation device. The nearest neighbor PSM technique was used. Propensity scores were estimated by unconditional logistic regression analyses that incorporated potential predictors of therapy as independent variables in the regression and group status (e.g., DET-S vs GLA-C) as the outcome. In Cohort 1, a 1 up to 5 ratio was applied to DET-S and GLA-C patients. This was done separately for the IMPACT® and Humana® databases. For Cohort 2, a 1 up to 2 ratio was applied to DET-C and GLA-S patients, with the two independent databases being pooled together. These ratios and matchings were chosen because our preliminary analysis revealed a prescription imbalance, with significantly more eligible patients in the insulin glargine group when compared with the insulin detemir group, and a much lower number of patients in the switcher group as compared to the continuers group. Additionally, one-to-many matching has previously been validated as a method to increase precision in cohort studies when compared with one-to-one matching, and has also been supported in a recent review assessing the quality of statistical methodologies in matched case–control studies [25, 26]. Matching was implemented without replacement and any patient without at least one match was excluded from the analysis. Between-group covariate balance was evaluated using descriptive t tests and χ 2 tests (with corresponding P values) and standardized differences, where a standardized difference <10 indicated adequate balance [27].
All analyses were performed using the intention-to-treat (ITT) approach on the matched patients. Due to the study design, DET-C or GLA-C patients could not, respectively, switch to insulin glargine or detemir during the follow-up period. However, GLA-S and DET-S patients were able to switch back to insulin detemir or glargine during the follow-up period. Among matched patients, clinical and economic outcomes during the 1 year of follow-up were summarized and compared, with P values provided by Student’s t test or χ 2 test, as appropriate. Since not every patient had HbA1c values at the end of the follow-up, as sensitivity analysis, change in 1-year follow-up HbA1c was estimated by generalized linear regression to adjust for potential baseline differences. Furthermore, 1-year follow-up HbA1c was estimated using a last-observation-carry-forward (LOCF) approach where, among those patients who did not have an HbA1c value at the end of follow-up, the last HbA1c value dated after the first-quarter of the 1-year follow-up was used. A sensitivity analysis was conducted using 1:1 PSM between DET-S and GLA-C patients in Cohort 1 and between GLA-S and DET-C patients in Cohort 2.
All analyses were conducted using SAS 9.3 statistical software (SAS Institute Inc., Cary, USA).
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
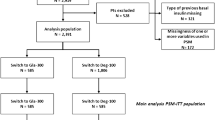
A total of 13,882 eligible US patients were identified in Cohort 1 (IMPACT®: DET-S n = 581 and GLA-C n = 8,094; Humana®: DET-S n = 277 and GLA-C n = 4,930; Fig. 1a) and 3,590 eligible patients in Cohort 2 (GLA-S n = 458 and DET-C n = 3,132; Fig. 1b). Prior to PSM, switcher patients were generally ‘sicker’ than patients who continued, with higher baseline HbA1c, more comorbidities, higher rate of RAI use, higher rate of hospitalization, and higher healthcare costs (Supplemental Table 1).
Patient attrition for Cohort 1 (a) and Cohort 2 (b). HbA 1c glycated hemoglobin, DET-C patients continuing on insulin detemir, DET-S patients switching from insulin glargine to insulin detemir, GLA-C patients continuing on insulin glargine, GLA-S patients switching from insulin detemir to insulin glargine, T2DM type 2 diabetes mellitus
After PSM, most of the eligible switcher patients were matched to the continuer patients with similar baseline demographic, clinical, and economic characteristics. Standardized differences between groups were all <10.
In Cohort 1, the final study population included 792 DET-S patients and 3,930 GLA-C patients (IMPACT®: n = 536 DET-S matched to n = 2,668 GLA-C, mean age 54 years old, 47.2% female, baseline HbA1c 8.6%; Humana®: n = 256 DET-S matched to n = 1,262 GLA-C, mean age 72.9 years old, 55% female, baseline HbA1c 8.2%; Table 1); patients in the Humana® database were older due to the fact that they were all required to be aged 65+ and covered by Medicare Advantage to be included in Cohort 1. Cohort 2 included 1,199 patients with a mean age of 60.8 years, 50.8% female and baseline HbA1c 8.94% (n = 419 GLA-S matched to n = 780 DET-C, Table 1).
Insulin Utilization
During the 1-year follow-up period, in Cohort 1, patients switching from insulin glargine to insulin detemir, compared with patients continuing on insulin glargine, showed significantly lower rates of index basal insulin treatment persistence, shorter duration of persistence (Fig. 2a, b) and lower rate of adherence (Fig. 3a, b). These findings were consistent in both commercially insured IMPACT® population, and Medicare-insured Humana® elderly population. In contrast, in Cohort 2, GLA-S patients, as compared with DET-C patients, showed numerically, but not statistically significantly, higher index basal insulin treatment persistence (Fig. 2a, b), and significantly higher rates of treatment adherence (Fig. 3a, b).
Treatment persistence after 1-year follow-up: the percentage of patients persistent with treatment (a), and the number of days between the index date and date of treatment discontinuation (b). DET-C patients continuing on insulin detemir, DET-S patients switching from insulin glargine to insulin detemir, GLA-C patients continuing on insulin glargine, GLA-S patients switching from insulin detemir to insulin glargine
Follow-up treatment adherence: the non-adjusted and adjusted MPRs for insulin switchers and insulin continuers (a), and the percentage of patients achieving non-adjusted and adjusted MPRs ≥0.8 for insulin switchers and insulin continuers (b). DET-C patients continuing on insulin detemir, DET-S patients switching from insulin glargine to insulin detemir, GLA-C patients continuing on insulin glargine, GLA-S patients switching from insulin detemir to insulin glargine, MPR medication possession ratio
The DACON was similar for DET-S and GLA-C patients (Fig. 4). During the follow-up, RAI utilization was significantly lower among GLA-C patients versus DET-S in Cohort 1, but similar between the GLA-S and DET-C groups in Cohort 2 (Fig. 5). Additionally, a significant proportion of DET-S patients (IMPACT®: 33.3%; Humana®: 40.2%) restarted insulin glargine sometime during the follow-up year. In contrast, in the GLA-S group, only 83 of 419 patients (19.8%) restarted insulin detemir during the follow-up period.
Follow-up DACON for insulin switchers and insulin continuers. DACON daily average consumption, DET-C patients continuing on insulin detemir, DET-S patients switching from insulin glargine to insulin detemir, GLA-C patients continuing on insulin glargine, GLA-S patients switching from insulin detemir to insulin glargine
Follow-up RAI use of insulin switchers and insulin continuers. DET-C patients continuing on insulin detemir, DET-S patients switching from insulin glargine to insulin detemir, GLA-C patients continuing on insulin glargine, GLA-S patients switching from insulin detemir to insulin glargine, RAI rapid-acting insulin
Clinical Outcomes
At the end of the follow-up year, in Cohort 1, mean HbA1c was significantly higher among DET-S patients compared with GLA-C patients (Fig. 6a), and the mean change in HbA1c from baseline was lower in the DET-S group than GLA-C group (only statistically significant in the IMPACT® database) (Fig. 6b). Significantly more GLA-C patients achieved a target HbA1c < 7.0% in the Humana® group and a target HbA1c < 8.0% in both the IMPACT® and Humana® groups (Fig. 6c).
HbA1c (a), change in HbA1c (b) and percentage of patients reaching HbA1c targets <7% and <8% (c) of insulin switchers and insulin continuers. HbA 1c glycated hemoglobin, DET-C, patients continuing on insulin detemir, DET-S patients switching from insulin glargine to insulin detemir, GLA-C patients continuing on insulin glargine, GLA-S patients switching from insulin detemir to insulin glargine
In Cohort 2, although mean HbA1c was not significantly different at the end of follow-up (Fig. 6a), a greater reduction from baseline in HbA1c value was achieved among GLA-S patients when compared with DET-C patients (Fig. 6b). Additionally, in the GLA-S group, at the end of 1-year follow-up, compared with those patients who remained on insulin glargine, those who restarted insulin detemir had higher HbA1c (8.86% vs 8.23%, P = 0.0539) and lower HbA1c reduction from baseline (−0.10% vs −0.81%; P = 0.0172). More patients in the GLA-S group achieved HbA1c goals during follow-up than did those in the DET-C group (Fig. 6c).
Although for Cohort 1 overall hypoglycemia prevalence rates were significantly higher for GLA-C in the Humana® database, overall both prevalence and event rates for Cohort 1 and 2 were similar between switchers and continuers, as were severe hospital/ED-related hypoglycemia rates (Table 2).
Economic Outcomes
Overall healthcare costs were similar across all four treatment cohorts (Table 3). Although healthcare utilization did not differ according to treatment in Cohort 1, DET-S patients had significantly higher diabetes drug (P < 0.0001) and diabetes supply costs (P = 0.0006) than GLA-C patients in the IMPACT® cohort; while in the Humana® cohort only diabetes drug costs were significantly higher for DET-S patients than for GLA-C patients (P = 0.0368; Table 3).
Total healthcare expenditure was $21,845 in the DET-C cohort and $24,417 in the GLA-S cohort and total expenditure for diabetes-related healthcare was $10,293 and $10,619, respectively. These differences were not statistically significant.
Sensitivity Analysis
For index basal insulin treatment persistence, both 75th and 95th percentiles of the refill time were used to estimate length of persistence and yielded similar results. For follow-up HbA1c analysis, generalized linear regression was used to estimate the changes in HbA1c from baseline, and the LOCF approach was also employed. Both approaches yielded similar results on both HbA1c levels and proportions of patients achieving glycemic targets as the primary analysis. Finally, PSM was also conducted using 1:1 ratio and overall similar results were observed (data not shown).
Discussion
This real-world US study investigated the effects of switching from insulin glargine to insulin detemir (Cohort 1) or from insulin detemir to insulin glargine (Cohort 2), compared with not switching and remaining on baseline treatment. In Cohort 1, of almost 14,000 T2DM patients from two independent national managed care populations (IMPACT® and Humana®) who had been treated with insulin glargine, only a small minority (6%) switched to insulin detemir. After matching baseline demographic and disease characteristics between GLA-C and DET-S, GLA-C patients had significantly better persistence and adherence with their long-acting insulin treatment compared with DET-S patients during 1 year of follow-up. Additional use of RAI was significantly higher among DET-S patients during the follow-up year, and 33–40% of DET-S patients restarted insulin glargine. DET-S patients in Cohort 1 had a significantly higher follow-up HbA1c, smaller HbA1c change, and lower glycemic target achievement compared with GLA-C patients. Although hypoglycemia prevalence rates were higher for GLA-C patients from the Humana® group, when compared with DET-S patients, rates were similar overall. Rates of more severe inpatient/ED-related hypoglycemia were low, and similar, in both groups. Healthcare utilization and total costs were also similar in both groups, but the DET-S group had higher diabetes drug and supply costs than did the GLA-C group. Similar results were generally found in the younger population of commercially insured patients (IMPACT® cohort) and the older Medicare population (Humana® cohort).
In Cohort 2, contrasting results were observed, GLA-S patients were more adherent during 1 year of follow-up, had greater HbA1c reduction from baseline, and had a significant increase in likelihood of achieving HbA1c goal <8% when compared with DET-C patients. The improved outcomes observed in the GLA-S group were achieved with similar rates of hypoglycemia, heath care utilization, and costs as in the DET-C group.
These data support previously published studies that show a low incidence of hypoglycemia among patients with T2DM treated with either insulin glargine or insulin detemir [4, 5, 15]. Similar to the PREDICTIVE trial [13], the prevalence rate of hypoglycemia was lower after switching from insulin glargine to insulin detemir in the Medicare cohort in our study; however, the event rates were similar in both groups. No difference in hypoglycemia was observed in the commercially insured IMPACT® population. In contrast to previously published studies showing that insulin dose was typically higher with insulin detemir than insulin glargine [5, 15, 28], it was similar among the matched GLA-C and DET-S patients based on the DACON estimate from the pharmacy claims data. In our study, however, DET-S patients had a much higher rate of RAI use than did GLA-C patients. Although dosing frequency information was not available for our data analysis, existing literature suggests that insulin detemir is more likely to be dosed more frequently than insulin glargine [15, 29]. Both higher twice-daily use and RAI use may explain the lower persistence and adherence rates and higher diabetes drug and supply costs in the DET-S group.
Data from Cohort 1 and Cohort 2 suggest that continued use of insulin glargine, as opposed to switching to insulin detemir, or switching from insulin detemir to insulin glargine, is associated with improved glycemic control, despite similar baseline HbA1c levels in both groups and higher RAI use in the DET-S group during the follow-up period. Although statistically significant, the difference between the basal insulins with regard to HbA1c reduction from baseline was 0.32% for GLA-S vs DET-C and 0.2% for GLA-C vs DET-S. The clinical relevance of differences of such magnitudes is unclear. Nonetheless, up to 8.7% more GLA-S patients achieved a target HbA1c < 8% during follow-up and as many as 8.0% more GLA-C patients achieved an HbA1c < 7% compared with DET-S. When one considers the enormity of the T2DM pandemic, such increases could represent a large number of patients. Furthermore, reductions in HbA1c of 0.9% are associated with a significant reduction in the risk of microvascular complications associated with T2DM and a reduction in HbA1c of just 0.6% leads to a reduction in risk of myocardial infarction and of diabetes-related and all-cause mortality [1].
The improvements in glycemic control observed in the current study conflict with results from the PREDICTIVE trial [13, 14] but are consistent with those from two US studies [15, 16]. In our study, the treatment persistence rate was significantly higher in the GLA-C group and medication adherence was significantly higher among GLA-S patients. Importantly, a positive association between HbA1c reduction and treatment persistence among T2DM patients receiving insulin therapy has previously been shown [7]. Also, similar to the results from the RELISH study [16], a significant portion of DET-S patients restarted insulin glargine after switching to insulin detemir. In contrast, 19.8% of GLA-S restarted insulin detemir during the follow-up period. Of note, compared to those patients who remained on insulin glargine, those who restarted insulin detemir had higher HbA1c values during follow-up and a lower HbA1c reduction from baseline.
Interpretations from this study are, however, limited due to the retrospective nature of the analysis. Although PSM was used to balance the observed baseline differences between the cohorts, unobserved selection bias may still exist, and thus causality cannot be established for the treatments and observed differences in outcomes. Indeed, since the unmatched groups show distinct differences, caution should be taken in generalizing these findings from managed care populations to all patients with T2DM. Data on reasons for switching and treatment satisfaction were not available, nor was information on the exact benefit design and physicians’ and patients’ preferences. The random-date approach to setting the index date for insulin continuers has been used in other studies [30, 31]; however, by definition, insulin continuers are “persistent” users at index date and, therefore, their follow-up persistence rate may be overestimated as compared to switchers. On the other hand, the fact that insulin continuers’ index dates were randomly selected between the third and the last study drug prescription date could imply underestimation of their persistence rate if dates from later prescriptions were selected. Additionally, patients with randomly assigned index dates may have differed from other patients in their unmeasured treatment needs and willingness to engage in certain treatment decisions [32]. The direction of the overall bias is hard to estimate due to these opposing possible sources of bias, but is believed to affect both GLA-C and DET-C patients in the current study. Data within claims databases may have inaccuracies introduced through coding errors that could not be identified because of a lack of access to additional patient records. Insulin persistence and adherence and daily dose were based on pharmacy claims data, which reflect prescription filling and do not verify that filled prescriptions were taken as directed, and also cannot account for amount of wastage. Furthermore, information on dosing frequency was not available, and recommended expiration periods are different between insulin glargine and detemir. Therefore, patients who followed the recommendations and were on a low dose may not have used the full amount in the vial or pen before discarding the remaining contents, leading to different estimates for DACON and adherence. While the targets used for HbA1c in the current study are often used for adults with T2DM, targets for individual patients are based on unique characteristics and may differ from these typical targets. Thus, some of the patients not reaching HbA1c < 7.0% or <8.0% may have had clinical targets that were higher than these typical targets. Also, data on patient body weight—an additional and important outcome—were not available for inclusion in this analysis. Baseline HbA1c was one of the cohort eligibility requirements, and we did not compare patients who had HbA1c available at baseline with those who had not. Finally, blood glucose data were not available, and identification of hypoglycemia events was based on ICD-9-CM codes in the claims data and was, therefore, subject to coding error. The true rate of hypoglycemia among the patients analyzed in our study may, however, be higher than that recorded in the claims database. Hypoglycemia event rates reported from claims databases may not provide sufficient information on hypoglycemia events compared with clinical trials, in which all instances of hypoglycemia are captured. In our study, due to a lack of glucose reading, we used the setting of hypoglycemia diagnosis as proxy of severity, with inpatient/ED hypoglycemia indicating severe hypoglycemia. This may result in further under-reporting of severe hypoglycemia rates. All the above-mentioned limitations restrict the generalizability of this study.
It should also be considered that most insulin-treated patients self-adjust insulin doses based on a number of factors such as current glucose levels, physical exercise, food consumption, etc. The accuracy of such fine dose adjustments depends on previous experience. Thus, when a patient is switched from an insulin they are familiar with, to another with different kinetic characteristics, one may expect a small deterioration of glycemic control and/or increase in hypoglycemic rates in the first few months. However, the goal of the current analysis was to study the clinical and economic trends of continuing treatment with insulin detemir or insulin glargine versus switching. Future studies should assess more closely dosing frequencies, the reasons for switching, etc.
Our study examined issues similar to those of previously conducted small-scale US studies of patients switching from insulin glargine to insulin detemir [15, 16]. However, the major strengths of our study compared to previous studies included that we examined a much larger study population from two independent cohorts, and that stringent PSM was used to balance the baseline characteristics of patients, thereby reducing potential confounders when interpreting results. Overall, the findings of this study support the use of insulin glargine, whether through continuation or switching, and may call for a careful re-examination of the therapeutic interchangeability of the two basal insulin analogs in the real-world setting. Indeed, these findings suggest that switching patients from insulin glargine to insulin detemir is more disruptive than switching patients from insulin detemir to insulin glargine. However, caution also needs to be exercised when interpreting these results due to the retrospective nature of this analysis.
Conclusion
In summary, the results from this study suggest that, in a real-world US managed care setting, switching patients with T2DM, including the elderly, from insulin detemir to insulin glargine, or maintaining patients on insulin glargine rather than switching to insulin detemir, may improve treatment persistence/adherence and enhance glycemic outcomes. This is achieved without implications regarding hypoglycemia or healthcare costs. These results suggest that, in the real-world setting, the long-acting basal insulin formulations, insulin glargine and insulin detemir, may not be therapeutically interchangeable. Further prospective studies, such as randomized pragmatic trials with a cross-over design, are needed to confirm the therapeutic non-interchangeability of insulin glargine and insulin detemir.
References
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55:1577–96.
Baxter MA. The role of new basal insulin analogues in the initiation and optimisation of insulin therapy in type 2 diabetes. Acta Diabetol. 2008;45:253–68.
Tsai ST, Pathan F, Ji L, et al. First insulinization with basal insulin in patients with Type 2 diabetes in a real-world setting in Asia. J Diabetes. 2011;3:208–16.
Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther. 2008;30:1976–87.
Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;CD006383.
Meneghini L, Kesavadev J, Demissie M, Nazeri A, Hollander P. Once-daily initiation of basal insulin as add-on to metformin: a 26-week, randomized, treat-to-target trial comparing insulin detemir with insulin glargine in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:729–36.
Xie L, Wei W, Pan C, Du J, Baser O. A real-world study of patients with type 2 diabetes initiating basal insulins via disposable pens. Adv Ther. 2011;28:1000–11.
Davis KL, Tangirala M, Meyers JL, Wei W. Real-world comparative outcomes of US type 2 diabetes patients initiating analog basal insulin therapy. Curr Med Res Opin. 2013;29:1083–91.
Borah BJ, Darkow T, Bouchard J, Aagren M, Forma F, Alemayehu B. A comparison of insulin use, glycemic control, and health care costs with insulin detemir and insulin glargine in insulin-naive patients with type 2 diabetes. Clin Ther. 2009;31:623–31.
McAdam-Marx C, Bouchard J, Aagren M, Nelson R, Brixner D. Analysis of glycaemic control and weight change in patients initiated with human or analog insulin in an US ambulatory care setting. Diabetes Obes Metab. 2010;12:54–64.
King AB. Once-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover study. Diabetes Obes Metab. 2009;11:69–71.
Lüddeke HJ, Sreenan S, Aczel S, PREDICTIVE Study Group, et al. PREDICTIVE—a global, prospective observational study to evaluate insulin detemir treatment in types 1 and 2 diabetes: baseline characteristics and predictors of hypoglycaemia from the European cohort. Diabetes Obes Metab. 2007;9:428–34.
Yenigun M, Honka M. Switching patients from insulin glargine-based basal-bolus regimens to a once daily insulin detemir-based basal-bolus regimen: results from a subgroup of the PREDICTIVE study. Int J Clin Pract. 2009;63:425–32.
Yang L, Christensen T, Sun F, Chang J. Cost-effectiveness of switching patients with Type 2 diabetes from insulin glargine to insulin detemir in Chinese setting: a health economic model based on the PREDICTIVE study. Value Health. 2012;15(suppl 1):S56–9.
Bryant GA, McDanel DL, Horner KE, Farris KB, Newkirk EN. Evaluation of dosing and clinical outcomes in patients undergoing conversion of insulin glargine to insulin detemir. Pharmacotherapy. 2013;33:56–62.
Chou J, Chu M, Kim K, Lui K, Teng C, Yang J, Yeganeh M. Retrospective evaluation of a long-acting insulin switch on hemoglobin A1C study: glargine to detemir (RELISH). J Manag Care Pharm. 2013;19:185.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), 2013. http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed 30 April 2013.
Baser O, Wei W, Baser E, Xie L. Clinical and economic outcomes in patients with type 2 diabetes initiating insulin glargine disposable pen versus exenatide BID. J Med Econ. 2011;14:673–80.
Levin P, Wei W, Wang L, Pan C, Douglas D, Baser O. Combination therapy with insulin glargine and exenatide: real-world outcomes in patients with type 2 diabetes. Curr Med Res Opin. 2012;28:439–46.
Xie L, Zhou S, Wei W, Gill J, Pan C, Baser O. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15:230–6.
Baser O, Bouchard J, DeLuzio T, Henk H, Aagren M. Assessment of adherence and health care costs of insulin device (FlexPen) versus conventional vial/syringe. Adv Ther. 2010;27:94–104.
American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–63.
NCQA Diabetes Recognition Program. http://www.ncqa.org/Programs/Recognition/DiabetesRecognitionProgramDRP.aspx. Accessed October 2013.
Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4.
Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69–80.
Niven DJ, Berthiaume LR, Fick GH, Laupland KB. Matched case-control studies: a review of reported statistical methodology. Clin Epidemiol. 2012;4:99–110.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Wallace JP, Wallace JL, McFarland MS. Comparing dosing of basal insulin analogues detemir and glargine: is it really unit-per-unit and dose-per-dose? Ann Pharmacother. 2014;48:361–8.
Dailey G, Admane K, Mercier F, Owens D. Relationship of insulin dose, A1c lowering, and weight in type 2 diabetes: comparing insulin glargine and insulin detemir. Diabetes Technol Ther. 2010;12:1019–27.
Kan HJ, Song X, Johnson BH, Bechtel B, O’Sullivan D, Molta CT. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. Biomed Res Int. 2013;2013:808391.
Leider HL, Dhaliwal J, Davis EJ, Kulakodlu M, Buikema AR. Healthcare costs and nonadherence among chronic opioid users. Am J Manag Care. 2011;17:32–40.
Szymanski BR, Bohnert KM, Zivin K, McCarthy JF. Integrated care: treatment initiation following positive depression screens. J Gen Intern Med. 2013;28:346–52.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Sanofi US, Inc. (Bridgewater, USA). The authors received writing/editorial support in the preparation of this manuscript, provided by Pim Dekker, PhD, of Excerpta Medica (Amsterdam, The Netherlands), funded by Sanofi US, Inc.
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Wenhui Wei, Steve Zhou, Onur Baser, and Jasvinder Gill contributed to the concept and design of the study, interpretation of the data, and drafting of the manuscript.
Raymond Miao, Chunshen Pan, and Lin Xie assisted in the design of the study and data collection and contributed to the writing of the manuscript.
All authors read and approved the final manuscript.
Conflict of interest
Wenhui Wei is an employee of Sanofi US, Inc. Steve Zhou is an employee of Sanofi US, Inc. Raymond Miao is an employee of Sanofi US, Inc. Jasvinder Gill is an employee of Sanofi US, Inc. Onur Baser is an employee of STATinMED. Lin Xie is an employee of STATinMED. STATinMED received funding to carry out this work from Sanofi US, Inc. Chunshen Pan is an employee of PRO Unlimited, which received funding to carry out this work from Sanofi US, Inc.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wei, W., Zhou, S., Miao, R. et al. Much Ado About Nothing? A Real-World Study of Patients with Type 2 Diabetes Switching Basal Insulin Analogs. Adv Ther 31, 539–560 (2014). https://doi.org/10.1007/s12325-014-0120-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-014-0120-1