Abstract
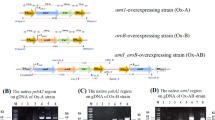
In a previous study, a putative 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase (ACMSD) was highly expressed in a mutant strain of Pyropia yezoensis, which exhibited an improved growth rate compared to its wild strain. To investigate the functional role of the putative ACMSD (Pyacmsd) of P. yezoensis, the putative Pyacmsd was cloned and expressed in Chlamydomonas reinhardtii. Recombinant C. reinhardtii cells with Pyacmsd (Cr_Pyacmsd) exhibited enhanced tolerance compared to control C. reinhardtii cells (Cr_control) under nitrogen starvation. Notably, Cr_Pyacmsd cells showed accumulation of lipids in nitrogen-enriched conditions. These results demonstrate the role of Pyacmsd in the generation of acetyl-coenzyme A. Thus, it can be used to enhance the production of biofuel using microalgae such as C. reinhardtii and increase the tolerance of other biological systems to nitrogen-deficient conditions.
Similar content being viewed by others
References
Bristow, L.A., Mohr, W., Ahmerkamp, S., and Kuypers, M.M. 2017. Nutrients that limit growth in the ocean. Curr. Biol. 27, R474–R478.
Cirulis, J.T., Strasser, B.C., Scott, J.A., and Ross, G.M. 2012. Optimization of staining conditions for microalgae with three lipophilic dyes to reduce precipitation and fluorescence variability. Cytometry A 81, 618–626.
de Lomana, A.L.G., Schäuble, S., Valenzuela, J., Imam, S., Carter, W., Bilgin, D.D., Yohn, C.B., Turkarslan, S., Reiss, D.J., Orellana, M.V., et al. 2015. Transcriptional program for nitrogen starvation-induced lipid accumulation in Chlamydomonas reinhardtii. Biotechnol. Biofuels 8, 207.
Garavaglia, S., Perozzi, S., Galeazzi, L., Raffaelli, N., and Rizzi, M. 2009. The crystal structure of human α-amino-β-carboxymuco-nate-s-semialdehyde decarboxylase in complex with 1, 3-dihydroxyacetonephosphate suggests a regulatory link between NAD synthesis and glycolysis. FEBS J. 276, 6615–6623.
Huo, L., Fielding, A.J., Chen, Y., Li, T., Iwaki, H., Hosler, J.P., Chen, L., Hasegawa, Y., Que, L.Jr., and Liu, A. 2012. Evidence for a dual role of an active site histidine in α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase. Biochemistry 51, 5811–5821.
Huo, L., Liu, F., Iwaki, H., Li, T., Hasegawa, Y., and Liu, A. 2015. Human α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD): a structural and mechanistic unveiling. Proteins 83, 178–187.
Im, S., Choi, S., Hwang, M.S., Park, E.J., Jeong, W.J., and Choi, D.W. 2015. De novo assembly of transcriptome from the gametophyte of the marine red algae Pyropia seriata and identification of abiotic stress response genes. J. Appl. Phycol. 27, 1343–1353.
Jeong, B., Wu-Scharf, D., Zhang, C., and Cerutti, H. 2002. Suppressors of transcriptional transgenic silencing in Chlamydomonas are sensitive to DNA-damaging agents and reactivate transposable elements. Proc. Natl. Acad. Sci. USA 99, 1076–1081.
Kamalanathan, M., Pierangelini, M., Shearman, L.A., Gleadow, R., and Beardall, J. 2016. Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J. Appl. Phycol. 28, 1509–1520.
Katsyuba, E., Mottis, A., Zietak, M., De Franco, F., van der Velpen, V., Gariani, K., Ryu, D., Cialabrini, L., Matilainen, O., Liscio, P., et al. 2018. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563, 354–359.
Kong, F., Cao, M., Sun, P., Liu, W., and Mao, Y. 2015. Selection of reference genes for gene expression normalization in Pyropia yezoensis using quantitative real-time PCR. J. Appl. Phycol. 27, 1003–1010.
Li, T., Iwaki, H., Fu, R., Hasegawa, Y., Zhang, H., and Liu, A. 2006. β-Amino-β-carboxymuconic-ε-semialdehyde decarboxylase (ACMSD) is a new member of the amidohydrolase superfamily. Biochemistry 45, 6628–6634.
Li, T., Walker, A.L., Iwaki, H., Hasegawa, Y., and Liu, A. 2005. Kinetic and spectroscopic characterization of ACMSD from Pseudomonas fluorescens reveals a pentacoordinate mononuclear metallocofactor. J. Am. Chem. Soc. 127, 12282–12290.
Lin, H., Kwan, A.L., and Dutcher, S.K. 2010. Synthesizing and salvaging NAD+: lessons learned from Chlamydomonas reinhardtii. PLoS Genet. 6, e1001105.
Mishra, S.K., Suh, W.I., Farooq, W., Moon, M., Shrivastav, A., Park, M.S., and Yang, J.W. 2014. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 155, 330–333.
Moore, C.M., Mills, M.M., Arrigo, K.R., Berman-Frank, I., Bopp, L., Boyd, P.W., Galbraith, E.D., Geider, R.J., Guieu, C., Jaccard, S.L., et al. 2013. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710.
Muraki, T., Taki, M., Hasegawa, Y., Iwaki, H., and Lau, P.C.K. 2003. Prokaryotic homologs of the eukaryotic 3-hydroxyanthranilate 3, 4-dioxygenase and 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase in the 2-nitrobenzoate degradation pathway of Pseudomonas fluorescens strain KU-7. Appl. Environ. Microbiol. 69, 1564–1572.
Na, Y., Lee, H.N., Wi, J., Jeong, W.J., and Choi, D.W. 2018. PtDRG1, a desiccation response gene from Pyropia tenera (Rhodophyta), exhibits chaperone function and enhances abiotic stress tolerance. Mar. Biotechnol. 20, 584–593.
Nakamura, Y., Sasaki, N., Kobayashi, M., Ojima, N., Yasuike, M., Shigenobu, Y., Satomi, M., Fukuma, Y., Shiwaku, K., Tsujimoto, A., et al. 2013. The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis). PLoS ONE 8, e57122.
Ngan Tran, K. and Choi, J. 2020. Comparative transcriptome analysis of high-growth and wild-type strains of Pyropia yezoensis. Acta Bot. Croat. 79, 148–156.
Palzer, L., Bader, J.J., Angel, F., Witzel, M., Blaser, S., McNeil, A., Wandersee, M.K., Leu, N.A., Lengner, C.J., Cho, C.E., et al. 2018. Alpha-amino-beta-carboxy-muconate-semialdehyde decarboxylase controls dietary niacin requirements for NAD+ synthesis. Cell Rep. 25, 1359–1370.
Park, S. and Choi, J. 2020. De novo transcriptome analysis of high growth rate Pyropia yezoensis (Bangiales, Rhodophyta) mutant with high utilization of nitrogen. Acta Bot. Croat. 79, 201–211.
Park, J.J., Wang, H., Gargouri, M., Deshpande, R.R., Skepper, J.N., Holguin, F.O., Juergens, M.T., Shachar-Hill, Y., Hicks, L.M., and Gang, D.R. 2015. The response of Chlamydomonas reinhardtii to nitrogen deprivation: a systems biology analysis. Plant J. 81, 611–624.
Rengel, R., Smith, R.T., Haslam, R.P., Sayanova, O., Vila, M., and León, R. 2018. Overexpression of acetyl-CoA synthetase (ACS) enhances the biosynthesis of neutral lipids and starch in the green microalga Chlamydomonas reinhardtii. Algal Res. 31, 183–193.
Sartory, D.P. and Grobbelaar, J.U. 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114, 177–187.
Shurubor, Y.I., D’Aurelio, M., Clark-Matott, J., Isakova, E.P., Deryabina, Y.I., Beal, M.F., Cooper, A.J.L., and Krasnikov, B.F. 2017. Determination of coenzyme A and acetyl-coenzyme A in biological samples using HPLC with UV detection. Molecules 22, 1388.
Wan, M., Rosenberg, J.N., Faruq, J., Betenbaugh, M.J., and Xia, J. 2011. An improved colony PCR procedure for genetic screening of Chlorella and related microalgae. Biotechnol. Lett. 33, 1615–1619.
Yoshino, J. 2019. ACMSD: a novel target for modulating NAD+ homeostasis. Trends Endocrinol. Metab. 30, 229–232.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2018R1D1A1B07049359), Golden Seed Project Grant funded by the Ministry of Oceans and Fisheries (213008-05-4-SB910), and the research program of Korea Atomic Energy Research Institute (KAERI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Park, Sj., Ahn, J.W. & Choi, Ji. Improved tolerance of recombinant Chlamydomonas rainhardtii with putative 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase from Pyropia yezoensis to nitrogen starvation. J Microbiol. 60, 63–69 (2022). https://doi.org/10.1007/s12275-022-1491-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-022-1491-7