Abstract
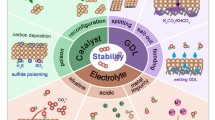
Developing efficient platinum-based electrocatalysts with super durability for the oxygen reduction reaction (ORR) is highly desirable to promote the large-scale commercialization of fuel cells. Although progress has been made in this aspect, the electrochemical kinetics and stability of platinum-based catalysts are still far from the requirements of the practical applications. Herein, PtPdFeCoNi high-entropy alloy (HEA) nanoparticles were demonstrated via a high-temperature injection method. PtPdFeCoNi HEA nanocatalyst exhibits outstanding catalytic activity and stability towards ORR due to the high entropy, lattice distortion, and sluggish diffusion effects of HEA, and the HEA nanoparticles delivered a mass activity of 1.23 A/mgPt and a specific activity of 1.80 mA/cmPt2, which enhanced by 6.2 and 4.9 times, respectively, compared with the values of the commercial Pt/C catalyst. More importantly, the high durability of PtPdFeCoNi HEA/C was evidenced by only 6 mV negative-shifted half-wave potential after 50,000 cycles of accelerated durability test (ADT).

Similar content being viewed by others
References
Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G. F.; Ross, P. N.; Lucas, C. A.; Marković, N. M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497.
Chen, C.; Kang, Y. J.; Huo, Z. Y.; Zhu, Z. W.; Huang, W. Y.; Xin, H. L.; Snyder, J. D.; Li, D. G.; Herron, J. A.; Mavrikakis, M. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343.
Zhu, Y. M.; Peng, J. H.; Zhu, X. R.; Bu, L. Z.; Shao, Q.; Pao, C. W.; Hu, Z. W.; Li, Y. F.; Wu, J. B.; Huang, X. Q. A large-scalable, surfactant-free, and ultrastable Ru-doped Pt3Co oxygen reduction catalyst. Nano Lett. 2021, 21, 6625–6632.
Yang, C. L.; Wang, L. N.; Yin, P.; Liu, J. Y.; Chen, M. X.; Yan, Q. Q.; Wang, Z. S.; Xu, S. L.; Chu, S. Q.; Cui, C. H. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464.
Chong, L.; Wen, J. G.; Kubal, J.; Sen, F. G.; Zou, J. X.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W. J.; Liu, D. J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281.
Li, X.; He, Y. H.; Cheng, S. B.; Li, B. Y.; Zeng, Y. C.; Xie, Z. H.; Meng, Q. P.; Ma, L.; Kisslinger, K.; Tong, X. et al. Atomic structure evolution of Pt-Co binary catalysts: Single metal sites versus intermetallic nanocrystals. Adv. Mater. 2021, 33, 2106371.
Wang, Q.; Zhao, Z. L.; Zhang, Z.; Feng, T. L.; Zhong, R. Y.; Xu, H.; Pantelides, S. T.; Gu, M. Sub-3 nm intermetallic ordered Pt3In clusters for oxygen reduction reaction. Adv. Sci. 2020, 7, 1901279.
Fan, X. J.; Peng, Z. W.; Ye, R. Q.; Zhou, H. Q.; Guo, X. M3C (M: Fe, Co, Ni) nanocrystals encased in graphene nanoribbons: An active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions. ACS Nano 2015, 9, 7407–7418.
Wu, Z. X.; Lv, Y. Y.; Xia, Y. Y.; Webley, P. A.; Zhao, D. Y. Ordered mesoporous platinum@graphitic carbon embedded nanophase as a highly active, stable, and methanol-tolerant oxygen reduction electrocatalyst. J. Am. Chem. Soc. 2012, 134, 2236–2245.
Kang, Y. J.; Li, M.; Cai, Y.; Cargnello, M.; Diaz, R. E.; Gordon, T. R.; Wieder, N. L.; Adzic, R. R.; Gorte, R. J.; Stach, E. A. et al. Heterogeneous catalysts need not be so “heterogeneous”: Monodisperse Pt nanocrystals by combining shape-controlled synthesis and purification by colloidal recrystallization. J. Am. Chem. Soc. 2013, 135, 2741–2747.
Cui, C. H.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771.
Zhang, H. F.; Qiu, X. Y.; Chen, Y. F.; Wang, S. Z.; Skrabalak, S. E.; Tang, Y. W. Shape control of monodispersed sub-5 nm Pd tetrahedrons and laciniate Pd nanourchins by maneuvering the dispersed state of additives for boosting ORR performance. Small 2020, 16, 1906026.
Wu, M. H.; Chen, C. L.; Zhao, Y. Z.; Zhu, E. B.; Li, Y. J. Atomic regulation of PGM electrocatalysts for the oxygen reduction reaction. Front. Chem. 2021, 9, 699861.
Su, K. Y.; Zhang, H. F.; Qian, S. Y.; Li, J. T.; Zhu, J. W.; Tang, Y. W.; Qiu, X. Y. Atomic crystal facet engineering of core-shell nanotetrahedrons restricted under sub-10 nanometer region. ACS Nano 2021, 15, 5178–5188.
Chang, F. F.; Shan, S. Y.; Petkov, V.; Skeete, Z.; Lu, A. L.; Ravid, J.; Wu, J. F.; Luo, J.; Yu, G.; Ren, Y. et al. Composition tunability and (111)-dominant facets of ultrathin platinum-gold alloy nanowires toward enhanced electrocatalysis. J. Am. Chem. Soc. 2016, 138, 12166–12175.
Kang, Y. J.; Snyder, J.; Chi, M. F.; Li, D. G.; More, K. L.; Markovic, N. M.; Stamenkovic, V. R. Multimetallic core/interlayer/shell nanostructures as advanced electrocatalysts. Nano Lett. 2014, 14, 6361–6367.
Xie, M. H.; Lyu, Z. H.; Chen, R. H.; Shen, M.; Cao, Z. M.; Xia, Y. N. Pt-Co@Pt octahedral nanocrystals: Enhancing their activity and durability toward oxygen reduction with an intermetallic core and an ultrathin shell. J. Am. Chem. Soc. 2021, 143, 8509–8518.
Tao, L.; Huang, B. L.; Jin, F. D.; Yang, Y.; Luo, M. C.; Sun, M. Z.; Liu, Q.; Gao, F. M.; Guo, S. J. Atomic PdAu interlayer sandwiched into Pd/Pt core/shell nanowires achieves superstable oxygen reduction catalysis. ACS Nano 2020, 14, 11570–11578.
Zhao, X.; Chen, S.; Fang, Z. C.; Ding, J.; Sang, W.; Wang, Y. C.; Zhao, J.; Peng, Z. M.; Zeng, J. Octahedral Pd@Pt18Ni core-shell nanocrystals with ultrathin PtNi alloy shells as active catalysts for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 2804–2807.
Greeley, J.; Stephens, I. E. L.; Bondarenko, A. S.; Johansson, T. P.; Hansen, H. A.; Jaramillo, T. F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J. K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556.
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Nørskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
Huang, X. Q.; Zhao, Z. P.; Cao, L.; Chen, Y.; Zhu, E. B.; Lin, Z. Y.; Li, M. F.; Yan, A. M.; Zettl, A.; Wang, Y. M. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234.
Bu, L. Z.; Zhang, N.; Guo, S. J.; Zhang, X.; Li, J.; Yao, J. L.; Wu, T.; Lu, G.; Ma, J. Y.; Su, D. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414.
Chung, D. Y.; Jun, S. W.; Yoon, G.; Kwon, S. G.; Shin, D. Y.; Seo, P.; Yoo, J. M.; Shin, H.; Chung, Y. H.; Kim, H. et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485.
Kong, Z. J.; Maswadeh, Y.; Vargas, J. A.; Shan, S. Y.; Wu, Z. P.; Kareem, H.; Leff, A. C.; Tran, D. T.; Chang, F. F.; Yan, S. et al. Origin of high activity and durability of twisty nanowire alloy catalysts under oxygen reduction and fuel cell operating conditions. J. Am. Chem. Soc. 2020, 142, 1287–1299.
Kang, Y. J.; Murray, C. B. Synthesis and electrocatalytic properties of cubic Mn−Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 2010, 132, 7568–7569.
Lopes, P. P.; Li, D. G.; Lv, H. F.; Wang, C.; Tripkovic, D.; Zhu, Y. S.; Schimmenti, R.; Daimon, H.; Kang, Y. J.; Snyder, J. et al. Eliminating dissolution of platinum-based electrocatalysts at the atomic scale. Nat. Mater. 2020, 19, 1207–1214.
Molaabasi, F.; Sarparast, M.; Shamsipur, M.; Irannejad, L.; Moosavi-Movahedi, A. A.; Ravandi, A.; Verdom, B. H.; Ghazfar, R. Shape-controlled synthesis of luminescent hemoglobin capped hollow porous platinum nanoclusters and their application to catalytic oxygen reduction and cancer imaging. Sci. Rep. 2018, 8, 14507.
Liu, J.; Li, W. Q.; Cheng, R. L.; Wu, Q.; Zhao, J. H.; He, D. P.; Mu, S. C. Stabilizing Pt nanocrystals encapsulated in N-doped carbon as double-active sites for catalyzing oxygen reduction reaction. Langmuir 2019, 35, 2580–2586.
Chen, L.; Leonardi, A.; Chen, J.; Cao, M. H.; Li, N.; Su, D.; Zhang, Q.; Engel, M.; Ye, X. C. Imaging the kinetics of anisotropic dissolution of bimetallic core-shell nanocubes using graphene liquid cells. Nat. Commun. 2020, 11, 3041.
Chen, S. P.; Niu, Z. Q.; Xie, C. L.; Gao, M. Y.; Lai, M. L.; Li, M. F.; Yang, P. D. Effects of catalyst processing on the activity and stability of Pt−Ni nanoframe electrocatalysts. ACS Nano 2018, 12, 8697–8705.
Su, F. B.; Poh, C. K.; Zeng, J. H.; Zhong, Z. Y.; Liu, Z. L.; Lin, J. Y. Pt nanoparticles supported on mesoporous carbon nanocomposites incorporated with Ni or Co nanoparticles for fuel cells. J. Power Sources 2012, 205, 136–144.
Sun, Y. F.; Dai, S. High-entropy materials for catalysis: A new frontier. Sci. Adv. 2021, 7, eabg1600.
Okejiri, F.; Yang, Z. Z.; Chen, H.; Do-Thanh, C. L.; Wang, T.; Yang, S. Z.; Dai, S. Ultrasound-driven fabrication of high-entropy alloy nanocatalysts promoted by alcoholic ionic liquids. Nano Res. 2022, 15, 4792–4798.
Wu, D. S.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Kitagawa, H. Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 2020, 142, 13833–13838.
Guo, R.; Yu, L. L.; Liu, Z. Y.; Pan, J.; Yao, Y. G.; Liu, L. Enthalpy induced phase partition toward hierarchical, nanostructured highentropy alloys. Nano Res. 2022, 15, 4893–4901.
Johny, J.; Li, Y.; Kamp, M.; Prymak, O.; Liang, S. X.; Krekeler, T.; Ritter, M.; Kienle, L.; Rehbock, C.; Barcikowski, S. et al. Lasergenerated high entropy metallic glass nanoparticles as bifunctional electrocatalysts. Nano Res. 2022, 15, 4807–4819.
Yao, Y. G.; Liu, Z. Y.; Xie, P. F.; Huang, Z. N.; Li, T. Y.; Morris, D.; Finfrock, Z.; Zhou, J. H.; Jiao, M. L.; Gao, J. L. et al. Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts. Sci. Adv. 2020, 6, eaaz0510.
Nie, S. Y.; Wu, L.; Zhao, L. C.; Zhang, P. F. Enthalpy-change driven synthesis of high-entropy perovskite nanoparticles. Nano Res. 2022, 15, 4867–4872.
Wang, Y.; Yu, B.; He, M.; Zhai, Z. H.; Yin, K. B.; Kong, F. G.; Zhang, Z. H. Eutectic-derived high-entropy nanoporous nanowires for efficient and stable water-to-hydrogen conversion. Nano Res. 2022, 15, 4820–4826.
Chen, J.; Zhou, X. Y.; Wang, W. L.; Liu, B.; Lv, Y. K.; Yang, W.; Xu, D. P.; Liu, Y. A review on fundamental of high entropy alloys with promising high-temperature properties. J. Alloys Compd. 2018, 760, 15–30.
Yao, Y. G.; Huang, Z. N.; Li, T. Y.; Wang, H.; Liu, Y. F.; Stein, H. S.; Mao, Y. M.; Gao, J. L.; Jiao, M. L.; Dong, Q. et al. High-throughput, combinatorial synthesis of multimetallic nanoclusters. Proc. Natl. Acad. Sci. USA 2020, 117, 6316–6322.
Chen, X. T.; Si, C. H.; Gao, Y. L.; Frenzel, J.; Sun, J. Z.; Eggeler, G.; Zhang, Z. H. Multi-component nanoporous platinum-ruthenium-copper-osmium-iridium alloy with enhanced electrocatalytic activity towards methanol oxidation and oxygen reduction. J. Power Sources 2015, 273, 324–332.
Yao, Y. G.; Huang, Z. N.; Xie, P. F.; Lacey, S. D.; Jacob, R. J.; Xie, H.; Chen, F. J.; Nie, A. M.; Pu, T. C.; Rehwoldt, M. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494.
Qiao, H. Y.; Saray, M. T.; Wang, X. Z.; Xu, S. M.; Chen, G.; Huang, Z. N.; Chen, C. J.; Zhong, G.; Dong, Q.; Hong, M. et al. Scalable synthesis of high entropy alloy nanoparticles by microwave heating. ACS Nano 2021, 15, 14928–14937.
Huang, X. Q.; Zhao, Z. P.; Fan, J. M.; Tan, Y. M.; Zheng, N. F. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J. Am. Chem. Soc. 2011, 133, 4718–4721.
Zhao, Z. L.; Zhang, L. Y.; Bao, S. J.; Li, C. M. One-pot synthesis of small and uniform Au@PtCu core-alloy shell nanoparticles as an efficient electrocatalyst for direct methanol fuel cells. Appl. Catal. B: Environ. 2015, 174–175, 361–366.
Huang, L. L.; Chen, P. C.; Liu, M. H.; Fu, X. B.; Gordiichuk, P.; Yu, Y. N.; Wolverton, C.; Kang, Y. J.; Mirkin, C. A. Catalyst design by scanning probe block copolymer lithography. Proc. Natl. Acad. Sci. USA 2018, 115, 3764–3769.
Li, Y. X.; Liao, Y. J.; Zhang, J.; Huang, E. H.; Ji, L. Z.; Zhang, Z. Y.; Zhao, R. Z.; Zhang, Z. M.; Yang, B.; Zhang, Y. H. et al. High-entropy-alloy nanoparticles with enhanced interband transitions for efficient photothermal conversion. Angew. Chem., Int. Ed. 2021, 60, 27113–27118.
Chen, W.; Dalach, P.; Schneider, W. F.; Wolverton, C. Interplay between subsurface ordering, surface segregation, and adsorption on Pt-Ti(111) near-surface alloys. Langmuir 2012, 28, 4683–4693.
Wang, G. W.; Huang, B.; Xiao, L.; Ren, Z. D.; Chen, H.; Wang, D. L.; Abruña, H. D.; Lu, J. T.; Zhuang, L. Pt skin on AuCu intermetallic substrate: A strategy to maximize Pt utilization for fuel cells. J. Am. Chem. Soc. 2014, 136, 9643–9649.
Zaman, S.; Su, Y. Q.; Dong, C. L.; Qi, R. J.; Huang, L.; Qin, Y. Y.; Huang, Y. C.; Li, F. M.; You, B.; Guo, W. et al. Scalable molten salt synthesis of platinum alloys planted in metal-nitrogen-graphene for efficient oxygen reduction. Angew. Chem. 2022, 134, e202115835.
Yang, Z. J.; Yang, H. Z.; Shang, L.; Zhang, T. R. Ordered PtFeIr intermetallic nanowires prepared through a silica-protection strategy for the oxygen reduction reaction. Angew. Chem. 2022, 134, e202113278.
Tian, X. L.; Zhao, X.; Su, Y. Q.; Wang, L. J.; Wang, H. M.; Dang, D.; Chi, B.; Liu, H. F.; Hensen, E. J. M.; Lou, X. W. et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856.
Li, M. F.; Zhao, Z. P.; Cheng, T.; Fortunelli, A.; Chen, C. Y.; Yu, R.; Zhang, Q. H.; Gu, L.; Merinov, B. V.; Lin, Z. Y. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419.
Impagnatiello, A.; Cerqueira, C. F.; Coulon, P. E.; Morin, A.; Escribano, S.; Guetaz, L.; Clochard, M. C.; Rizza, G. Degradation mechanisms of supported Pt nanocatalysts in proton exchange membrane fuel cells: An operando study through liquid cell transmission electron microscopy. ACS Appl. Energy Mater. 2020, 3, 2360–2371.
Shi, F. L.; Gao, W. P.; Shan, H.; Li, F.; Xiong, Y. L.; Peng, J. H.; Xiang, Q.; Chen, W. L.; Tao, P.; Song, C. Y. et al. Strain-induced corrosion kinetics at nanoscale are revealed in liquid: Enabling control of corrosion dynamics of electrocatalysis. Chem 2020, 6, 2257–2271.
Guo, S. J.; Zhang, S.; Sun, X. L.; Sun, S. H. Synthesis of ultrathin FePtPd nanowires and their use as catalysts for methanol oxidation reaction. J. Am. Chem. Soc. 2011, 133, 15354–15357.
Feng, Q. C.; Zhao, S.; He, D. S.; Tian, S. B.; Gu, L.; Wen, X. D.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Strain engineering to enhance the electrooxidation performance of atomic-layer Pt on intermetallic Pt3Ga. J. Am. Chem. Soc. 2018, 140, 2773–2776.
Acknowledgments
The work is supported by the National Natural Science Foundation of China (Nos. 21972016 and 21773023), National Youth Top-notch Talent Support Program of China, Sichuan Science and Technology Program (No. 2020YJ0243), Jiangsu Province Cultivation base for State Key Laboratory of Photovoltaic Science and Technology (No. SKLPST 202103), and Foundation of State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering (No. 2022-K28). We thank Dr. Pawan Kumar for help in TEM EDS characterization.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yu, Y., Xia, F., Wang, C. et al. High-entropy alloy nanoparticles as a promising electrocatalyst to enhance activity and durability for oxygen reduction. Nano Res. 15, 7868–7876 (2022). https://doi.org/10.1007/s12274-022-4432-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4432-1