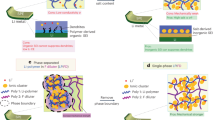
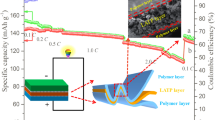
Abstract
The aggregation of inorganic particles with high mass ratio will form a heterogeneous electric field in the solid polymer electrolytes (SPEs), which is difficult to be compatible with lithium anode, leading to inadequate ionic conductivity. Herein, a facile spray drying method is adopted to increase the mass ratio of inorganic particles and solve the aggregation problems of fillers simultaneously. The polyvinylidene fluoride (PVDF) with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) covers the surface of each Li6.4La3Zr1.4Ta0.6O12 (LLZTO) granules during the nebulization process, then forming flat solid electrolytes via layer-by-layer deposition. Characterized by the atomic force microscope, the obtained solid electrolytes achieve a homogenous dispersion of Young’s modulus and surface electric field. As a result, the as-prepared SPEs present high tensile strength of 7.1 MPa, high ionic conductivity of 1.86 × 10−4 S·cm−1 at room temperature, and wide electrochemical window up to 5.0 V, demonstrating increased mechanical strength and uniform lithium-ion migration channels for SPEs. Thanks to the as-prepared SPEs, the lithium-symmetrical cells show a highly stable Li plating/stripping cycling for over 1,000 h at 0.1 mA·cm−2. The corresponding Li/LCoO2 batteries also present good rate capability and excellent cyclic performance with capacity retention of 80% after 100 cycles at room temperature.

Similar content being viewed by others
References
Cui, J.; Chen, X.; Zhou, Z.; Zuo, M.; Xiao, Y.; Zhao, N.; Shi, C.; Guo, X. Effect of continuous pressures on electrochemical performance of Si anodes. Mater. Today Energy 2021, 20, 100632.
Zhang, X. J.; Chen, L. L.; Yang, Z.; Qiu, X. Y.; Zheng, Z. M. Investigation of the structural effect of the carbon coated separator towards to the properties of Li-S batteries. Mater. Lett. 2021, 290, 129512.
Yang, Y.; Zhu, H.; Xiao, J. F.; Geng, H. B.; Zhang, Y. F.; Zhao, J. B.; Li, G.; Wang, X. L.; Li, C. C.; Liu, Q. Achieving ultrahigh-rate and high-safety Li+ storage based on interconnected tunnel structure in micro-size niobium tungsten oxides. Adv. Mater. 2020, 32, 1905295.
Shi, C.; Zhu, J. W.; Shen, X.; Chen, F. X.; Ning, F. G.; Zhang, H. D.; Long, Y. Z.; Ning, X.; Zhao, J. B. Flexible inorganic membranes used as a high thermal safety separator for the lithium-ion battery. RSC Adv. 2018, 8, 4072–4077.
Wang, C. W.; Fu, K.; Kammampata, S. P.; McOwen, D. W.; Samson, A. J.; Zhang, L.; Hitz, G. T.; Nolan, A. M.; Wachsman, E. D.; Mo, Y. F. et al. Garnet-type solid-state electrolytes: Materials, interfaces, and batteries. Chem. Rev. 2020, 10, 4257–4300.
Manthiram, A.; Yu, X. W; Wang, S. F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103.
Niu, C. J.; Lee, H.; Chen, S. R.; Li, Q. Y.; Du, J.; Xu, W.; Zhang, J. G.; Whittingham, M. S.; Xiao, J.; Liu, J. High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 2019, 4, 551–559.
Zhou, Q.; Dong, S. M.; Lv, Z. L.; Xu, G. J.; Huang, L.; Wang, Q. L.; Cui, Z. L.; Cui, G. L. A temperature-responsive electrolyte endowing superior safety characteristic of lithium metal batteries. Adv. Energy Mater. 2020, 10, 1903441.
Ren, X. C.; Zhang, X. Q.; Xu, R.; Huang, J. Q.; Zhang, Q. Analyzing energy materials by cryogenic electron microscopy. Adv. Mater. 2020, 32, 1908293.
Zhang, Q. Q.; Liu, K.; Ding, F.; Liu, X. J. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174.
Zhang, Z. Z.; Shao, Y. J.; Lotsch, B.; Hu, Y. S.; Li, H.; Janek, J.; Nazar, L. F.; Nan, C. W.; Maier, J.; Armand, M. et al. New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 2018, 11, 1945–1976.
Wu, B. B.; Wang, S. Y.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W. Q.; Yang, J. H.; Xiao, J. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 2018, 11, 1803–1810.
Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc 2005, 152, A396–A404.
Brissot, C.; Rosso, M. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 1999, 81–82, 925–929.
Hu, Y. R.; Zhong, Y. R.; Qi, L. M.; Wang, H. L. Inorganic/polymer hybrid layer stabilizing anode/electrolyte interfaces in solid-state Li metal batteries. Nano Res. 2020, 13, 3230–3234.
Han, F. D.; Westover, A. S.; Yue, J.; Fan, X. L.; Wang, F.; Chi, M. F.; Leonard, D. N.; Dudney, N. J.; Wang, H.; Wang, C. S. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 2019, 4, 187–196.
Cui, J.; Zhou, Z. H.; Jia, M. Y.; Chen, X.; Shi, C.; Zhao, N.; Guo, X. X. Solid polymer electrolytes with flexible framework of SiO2 nanofibers for highly safe solid lithium batteries. Polymers 2020, 12, 1324.
Lin, D. C.; Liu, W.; Liu, Y. Y.; Lee, H. R.; Hsu, P. C.; Liu, K.; Cui, Y. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide). Nano Lett. 2016, 16, 459–465.
Li, Y.; Zhang, W.; Dou, Q. Q.; Wong, K. W.; Ng, K. M. Li7La3Zr2O12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. J. Mater. Chem. A 2019, 7, 3391–3398.
Xie, H.; Yang, C. P.; Fu, K. K.; Yao, Y. G.; Jiang, F.; Hitz, E.; Liu, B. Y.; Wang, S.; Hu, L. B. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose. Adv. Energy Mater. 2018, 8, 1703474.
Chen, R. J.; Qu, W. J.; Guo, X.; Li, L.; Wu, F. The pursuit of solid-state electrolytes for lithium batteries: From comprehensive insight to emerging horizons. Mater. Horizons 2016, 3, 487–516.
Huo, H. Y.; Chen, Y.; Luo, J.; Yang, X. F.; Guo, X. X.; Sun, X. L. Rational design of hierarchical “ceramic-in-polymer” and “polymer-in-ceramic” electrolytes for dendrite-free solid-state batteries. Adv. Energy Mater. 2019, 9, 1804004.
Li, Y. T.; Xu, B. Y.; Xu, H. X.; Duan, H. N.; Lü, X. J.; Xin, S.; Zhou, W. D.; Xue, L. G.; Fu, G. T.; Manthiram, A. et al. Hybrid polymer/garnet electrolyte with a small interfacial resistance for lithium-ion batteries. Angew. Chem., Int. Ed. 2017, 56, 753–756.
Choi, H.; Kim, H. W.; Ki, J. K.; Lim, Y. J.; Kim, Y.; Ahn, J. H. Nanocomposite quasi-solid-state electrolyte for high-safety lithium batteries. Nano Res. 2017, 10, 3092–3102.
Bae, J.; Li, Y. T.; Zhao, F.; Zhou, X. Y.; Ding, Y.; Yu, G. H. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Mater. 2018, 15, 46–52.
Li, R. G.; Guo, S. T.; Yu, L.; Wang, L. B.; Wu, D. B.; Li, Y. Q.; Hu, X. L. Morphosynthesis of 3D macroporous garnet frameworks and perfusion of polymer-stabilized lithium salts for flexible solid-state hybrid electrolytes. Adv. Mater. Interfaces 2019, 6, 1900200.
Huang, Z. Y.; Pang, W. Y.; Liang, P.; Jin, Z. H.; Grundish, N.; Li, Y. T.; Wang, C. A. A dopamine modified Li6.4La3Zr1.4Ta0.6O12/PEO solid-state electrolyte: Enhanced thermal and electrochemical properties. J. Mater. Chem. A 2019, 7, 16425–16436.
Bae, J.; Li, Y. T.; Zhang, J.; Zhou, X. Y.; Zhao, F.; Shi, Y.; Goodenough, J. B.; Yu, G. H. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte. Angew. Chem., Int. Ed. 2018, 57, 2096–2100.
Palmer, M. J.; Kalnaus, S.; Dixit, M. B.; Westover, A. S.; Hatzell, K. B.; Dudney, N. J.; Chen, X. C. A three-dimensional interconnected polymer/ceramic composite as a thin film solid electrolyte. Energy Storage Mater. 2020, 26, 242–249.
Du, F. M.; Zhao, N.; Fang, R.; Cui, Z. H.; Li, Y. Q.; Guo, X. X. Influence of electronic conducting additives on cycle performance of garnet-based solid lithium batteries. J. Inorg. Mater. 2018, 33, 462–468.
Zhang, X.; Liu, T.; Zhang, S. F.; Huang, X.; Xu, B. Q.; Lin, Y. H.; Xu, B.; Li, L. L.; Nan, C. W.; Shen, Y. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785.
Le, H. T. T.; Ngo, D. T.; Kalubarme, R. S.; Cao, G. Z.; Park, C. N.; Park, C. J. Composite gel polymer electrolyte based on poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) with modified aluminum-doped lithium lanthanum titanate (A-LLTO) for high-performance lithium rechargeable batteries. ACS Appl. Mater. Interfaces 2016, 8, 20710–20719.
Shi, C.; Dai, J. H.; Huang, S. H.; Li, C.; Shen, X.; Zhang, P.; Wu, D. Z.; Sun, D. H.; Zhao, J. B. A simple method to prepare a polydopamine modified core-shell structure composite separator for application in high-safety lithium-ion batteries. J. Membrane Sci. 2016, 518, 168–177.
Leng, J.; Wang, Z. X.; Li, X. H.; Guo, H. J.; Yan, G. C.; Hu, Q. Y.; Peng, W. J.; Wang, J. X. A novel dried plum-like yolk-shell architecture of tin oxide nanodots embedded into a carbon matrix: Ultra-fast assembly and superior lithium storage properties. J. Mater. Chem. A 2019, 7, 5803–5810.
Li, Q.; Zhu, G. Z.; Zhao, Y. H.; Pei, K.; Che, R. C. NixMnyCozO nanowire/CNT composite microspheres with 3D interconnected conductive network structure via spray-drying method: A high-capacity and long-cycle-life anode material for lithium-ion batteries. Small 2019, 15, 1900069.
He, Z. J.; Chen, L.; Zhang, B. C.; Liu, Y. C.; Fan, L. Z. Flexible poly(ethylene carbonate)/garnet composite solid electrolyte reinforced by poly(vinylidene fluoride-hexafluoropropylene) for lithium metal batteries. J. Power Sources 2018, 392, 232–238.
Li, Z.; Huang, H. M.; Zhu, J. K.; Wu, J. F.; Yang, H.; Wei, L.; Guo, X. Ionic conduction in composite polymer electrolytes: Case of PEO: Ga-LLZO composites. ACS Appl. Mater. Interfaces 2019, 11, 784–791.
Du, F. M.; Zhao, N.; Li, Y. Q.; Chen, C.; Liu, Z. W.; Guo, X. X. All solid state lithium batteries based on lamellar garnet-type ceramic electrolytes. J. Power Sources 2015, 300, 24–28.
Li, Y. Q.; Wang, Z.; Li, C. L.; Cao, Y.; Guo, X. X. Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J. Power Sources 2014, 248, 642–646.
Gao, Z. H.; Sun, H. B.; Fu, L.; Ye, F. L.; Zhang, Y.; Luo, W.; Huang, Y. H. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702.
Thangadurai, V.; Weppner, W. Li6ALa2Ta2O12 (A = Sr, Ba): Novel garnet-like oxides for fast lithium ion conduction. Adv. Funct. Mater. 2005, 15, 107–112.
Kim, Y.; Yoo, A.; Schmidt, R.; Sharafi, A.; Lee, H.; Wolfenstine, J.; Sakamoto, J. Electrochemical stability of Li6.5La3Zr1.5M0.5O12 (M = Nb or Ta) against metallic lithium. Front. Energy Res. 2016, 4, 20.
Burnett, T.; Yakimova, R.; Kazakova, O. Mapping of local electrical properties in epitaxial graphene using electrostatic force microscopy. Nano Lett. 2011, 11, 2324–2328.
Panchal, V.; Pearce, R.; Yakimova, R.; Tzalenchuk, A.; Kazakova, O. Standardization of surface potential measurements of graphene domains. Sci. Rep. 2013, 3, 2597.
Liu, C.; Li, X.; Revilla, R. I.; Sun, T.; Zhao, J. B.; Zhang, D. W.; Yang, S. F.; Liu, Z. Y.; Cheng, X. Q.; Terryn, H. et al. Towards a better understanding of localised corrosion induced by typical non-metallic inclusions in low-alloy steels. Corrosion Sci. 2021, 179, 109150.
Zhang, X.; Wang, S.; Xue, C. J.; Xin, C. Z.; Lin, Y.; Shen, Y. H.; Li, L. L.; Nan, C. W. Response to comment on “self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly(vinylidene difluoride)-based solid electrolytes”. Adv. Mater. 2020, 32, 2000026.
Yamada, H.; Bhattacharyya, A. J.; Maier, J. Extremely high silver ionic conductivity in composites of silver halide (AgBr, AgI) and mesoporous alumina. Adv. Funct. Mater. 2006, 16, 525–530.
Li, M.; Guo, L. Q.; Qiao, L. J.; Bai, Y. The mechanism of hydrogen-induced pitting corrosion in duplex stainless steel studied by SKPFM. Corros. Sci. 2012, 60, 76–81.
Wei, L.; Liu, Y.; Li, Q.; Cheng, Y. F. Effect of roughness on general corrosion and pitting of (FeCoCrNi)0.89(WC)0.11 high-entropy alloy composite in 3.5 wt.% NaCl solution. Corros. Sci. 2019, 146, 44–57.
Jie, J.; Liu, Y. L.; Cong, L. N.; Zhang, B. H.; Lu, W.; Zhang, X. M.; Liu, J.; Xie, H. M.; Sun, L. Q. High-performance PVDF-HFP based gel polymer electrolyte with a safe solvent in Li metal polymer battery. J. Energy Chem. 2020, 49, 80–88.
Tan, S. J.; Zeng, X. X.; Ma, Q.; Wu, X. W.; Guo, Y. G. Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries. Electrochem. Energy Rev. 2018, 7, 113–138.
Zhu, Y. H.; Cao, J.; Chen, H.; Yu, Q. P.; Li, B. H. High electrochemical stability of a 3D cross-linked network PEO@nano-SiO2 composite polymer electrolyte for lithium metal batteries. J. Mater. Chem. A 2019, 7, 6832–6839.
Zheng, J.; Hu, Y. Y. New Insights into the compositional dependence of li-ion transport in polymer-ceramic composite electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 4113–4120.
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21805147).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_3683_MOESM1_ESM.pdf
A homogenous solid polymer electrolyte prepared by facile spray drying method is used for room-temperature solid lithium metal batteries
Rights and permissions
About this article
Cite this article
Zhou, Z., Sun, T., Cui, J. et al. A homogenous solid polymer electrolyte prepared by facile spray drying method is used for room-temperature solid lithium metal batteries. Nano Res. 16, 5080–5086 (2023). https://doi.org/10.1007/s12274-021-3683-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3683-6